Abstract
Purpose
To describe presented interaction alerts in older patients, and the extent to which these require further medical action for the specific patient or are already being addressed.
Methods
Interaction alerts presented at a physician consultation, for 274 consecutive primary care patients treated with two or more drugs (median age: 75 years; 59% female), were extracted. These alerts are based on Janusmed, a decision support integrated in the medical records that provides recommendations for managing the interactions. One general practitioner (GP) and one GP/clinical pharmacologist determined in retrospect, first independently and then in consensus, whether the alerts justified further medical action, considering each patient’s health condition.
Results
In all, 405 drug interaction alerts in 151 (55%) patients were triggered. Medical action in response was deemed medically justified for 35 (9%) alerts in 26 (17%) patients. These actions most often involved a switch to a less interacting drug from the same drug class (n = 10), a separate intake (n = 9), or the ordering of a laboratory test (n = 8). Out of 531 actions suggested by the alert system, only 38 (7%) were applicable to the specific patient, as, for instance, laboratory parameters were already being satisfactorily monitored or a separate intake implemented.
Conclusions
More than every other older patient receives drug treatment that triggers drug interaction alerts. Nine in ten alerts were already being addressed or were not relevant in the clinical setting, whereas, for the remaining tenth, some medical action, that for unknown reasons had not been taken, was reasonable. These findings show that interaction alerts are questionable as indicators of problematic prescribing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As patients are being treated with an increasing number of drugs [1], the risk of drug interactions is rising and interactions have been described as a significant cause of hospital visits and admissions [2]. It is, however, difficult to estimate the significance of drug interactions in clinical practice, as studies often report prevalence figures based solely on hits in interaction databases, and only rarely attempt to assess actual harm and other medical outcomes of interactions [3, 4].
Several web-based electronic databases have been developed to identify potentially problematic drug interactions [5, 6], forming the basis for clinical decision support systems [7,8,9]. Such systems have been shown to be useful during the patient consultation [10]. On the other hand, decision support systems pose a risk of alert fatigue, previously described as the mental state resulting from too many alerts consuming time and mental energy, which can cause important alerts to be ignored along with clinically unimportant ones [11]. A systematic review reported that up to 71% of hospitalised patients have potential drug interactions [3], and another review reported that the prevalence of clinically manifested interactions in hospitalised patients ranged from 1 to 64% [4]. Given these figures, it may not be surprising that up to 95% of drug interaction alerts have been reported to be overridden by clinicians [12]. There are various reasons for overriding an alert, one being that the potential interaction problem has already been taken care of. Unless an automatic decision support system is made aware of this by integration with patient data, it will keep on presenting alerts as long as the interacting drugs remain in the medication list. The extent to which automatically generated interaction alerts are already managed in health care, and details of such management, is an under-researched topic.
Given that the prevalence of drug interactions is used scientifically to reflect drug treatment quality, as illustrated by the recent inclusion of this item in core outcome sets for improving prescribing [13, 14], and, further, that the purpose of interaction alerts is to aid clinical decision making, there is a need for increased knowledge on interaction alerts in clinical practice. In this study, we aimed to shed light on interaction alerts in older patients, and the extent to which these require further action or are already being adequately addressed by the patient’s physician.
Methods
This descriptive study was conducted using data from a previous study investigating the association between medication reviews (recorded by a procedure code) and the adequacy of drug treatment management, in 302 consecutive patients (≥ 65 years of age) with a planned physician consultation at one of two Swedish primary care centres in the autumn of 2017 [15]. In that study, the drug treatment of each patient was retrospectively assessed by two specialist physicians (N.P.L., general practitioner (GP); S.S., GP/clinical pharmacologist), based on printouts from the electronic medical records over the 2½ years preceding the consultation, including laboratory tests, hospital discharge records, vaccinations, prescriptions, as well as interaction alerts originating from the Swedish national interaction database Janusmed [7]. The assessors determined whether an action related to the drug treatment was medically justified, prior to the next regular visit (see Appendix 1 for sentences guiding their categorisation). The assessments were performed from an overall medical perspective, first independently and then jointly where disagreements were resolved through consensus.
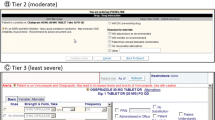
In the current study, patients with fewer than two drugs in the medication list were excluded; drugs for topical use were only counted if having potential systemic effects. Drugs used regularly or pro re nata (PRN) were considered. We recorded presented Janusmed category B, C and D interaction alerts: B, an interaction where the clinical relevance is uncertain or varies; C, a clinically relevant interaction that can be managed by either dose adjustments or separated intake; and D, a clinically relevant interaction where the recommendation is to avoid the drug combination [16]. These alerts are automatically presented in the prescribing module of the electronic health record system, as buttons highlighted in white, yellow and red, respectively. Therefore, the prescribing physicians were exposed to, and had the chance to react to, the same alerts as did the assessors. We also entered each patient’s current medication list into the open-access interface of Janusmed (January 2020) [17] to retrieve the specific recommendations provided to manage the alerts [7].
Additional medically justified actions prior to the next regular visit were recorded if they were related to the interaction alerts, as determined retrospectively by the assessors in consensus. For instance, this could include the switch or withdrawal of a drug, the ordering of a laboratory test, the retrieval of more information about the patient, or arranging an extra visit. Dosing was considered in the assessments.
Patients’ characteristics included age, sex, residence, and morbidities appearing in the Screening Tool of Older Persons’ Prescriptions (STOPP), the Screening Tool to Alert to Right Treatment (START), or the Swedish set of indicators of prescribing quality provided by the National Board of Health and Welfare [18, 19]. Multi-dose drug dispensing, i.e. machine-dispensed unit bags with drugs that are ingested at specific times of the day, intended for patients with difficulties in handling their drugs, has been associated with D interactions [20]. Therefore, we also recorded whether the patient was using this system or not.
Ethics approval
The study was approved by the Regional Ethical Review Board in Gothenburg, Sweden (DRN: 1046–15).
Statistics
We performed descriptive analyses using SPSS for Windows, version 24.0 (IBM SPSS, Armonk, NY, USA). The inter-rater agreement was assessed using kappa statistics.
Results
In all, 274 patients were included in the analysis (Fig. 1, Table 1). The median age was 75 years and 163 (59%) were women. The patients were treated with a median of seven drugs, ranging from two to 20. A total of 33 out of 274 (12%) patients were using multi-dose drug dispensing.
The interaction decision support system presented 405 drug interaction alerts, encompassing 185 unique drug-drug combinations, in 151 (55%) patients (Table 2). Up to 13 drug combination alerts were detected in a single individual. Overall, an additional action was deemed medically justified for 35 (9%) alerts in 26 patients (9% of all patients, 17% of patients with one or more interaction alerts). For 349 (86%) of 405 alerts, the assessors made the same assessment regarding whether a related action was medically justified or not, resulting in a kappa value of 0.44.
Joined to the 405 alerts, Janusmed provided a total of 531 recommendations, 38 (7%) of which were judged applicable to the specific patient (Table 3). None of the recommendations to monitor clinical signs, to perform therapeutic drug monitoring (TDM), and to add a proton pump inhibitor (PPI) required any action. Other specific Janusmed recommendations were applicable in 2–40% of the cases in which they were provided.
Medically justified actions related to drug interactions primarily concerned switching to a less interacting drug with the same mechanism of action (n = 10), separating the intake (n = 9), ordering a laboratory test (n = 8), or searching for more information before decision making (n = 5) (Table 4). Interaction alerts where a related action was medically justified frequently involved omeprazole (n = 9), (es)citalopram (n = 9), clopidogrel (n = 6), ferrous sulphate (n = 5), spironolactone (n = 4) or furosemide (n = 3). Four of these included a drug used PRN (diclofenac, n = 3; codeine, n = 1).
A total of nine D interaction alerts were presented in eight (3%) patients, all with unique drug combinations. For four of these alerts, encountered in four (1%) patients, some additional action was deemed medically justified (Table 4). Two actions involved consulting the patient’s cardiologist, one concerned a suggestion to switch to a less interacting drug within the same pharmacological subgroup, and one involved the withdrawal of a drug.
Category C interaction alerts were presented in 101 (37%) individuals. For 31 (16%) out of a total of 197 alerts, in 22 (8%) individuals, some additional action was considered medically justified. A frequent reason for the assessment that no action was needed, was that at least one of the drugs was used PRN (41 alerts in 26 individuals). Another frequent reason was ongoing monitoring of relevant laboratory parameters with adequate findings, for instance thyroid-stimulating hormone (TSH) in interactions involving levothyroxine (20 out of 23 alerts); international normalised ratio (INR) in interactions involving warfarin (20 out of 20 alerts); or electrolytes in drug combinations affecting sodium and/or potassium levels (ten out of 17 alerts). In addition, patients had usually already been informed about separated intake (25 out of 32 alerts). None of category B interaction alerts were considered to warrant medical action.
In 26 (79%) out of 33 patients using multi-dose drug dispensing, with a mean of twelve drugs in the medication list (range: four to 20), a total of 44 C, and 34 B interaction alerts were presented. In all, the assessors considered additional action to be medically justified in ten (13%) of these 78 alerts, all in the C category and encountered in seven (21%) patients. These actions involved a change to pantoprazole (n = 5), separated intake (n = 3), and the ordering of a laboratory test (n = 2).
Discussion
In this study, we show that more than every second older patient with two or more drugs in their medication list has drug treatment that causes alerts in a well-established drug interaction decision support system. Only one alert out of eleven was considered medically justified to act upon prior to the next regular visit; in these cases, the prescribing physician had, for unknown reason, not taken action. The remaining alerts were either already being addressed or were not relevant in the clinical setting.
One interpretation of our results is that the interaction alerts integrated as a decision support tool have had the intended effect, that is, to affect clinicians' behaviour. For instance, the monitoring of laboratory parameters and TDM seem to be well managed in most cases, as seems to be the monitoring of clinical parameters including blood pressure, electrocardiogram and weight. Similarly, recommendations to adjust the dose, a strategy to mitigate potentially adverse consequences caused by drug interactions, merited action in merely one out of 50 alerts providing such advice. Our findings are in tune with previous research demonstrating that the prevalence of D interaction alerts decreased following the introduction of the interaction alert system used in the present study [16]; that interaction alerts are appropriately overridden in up to 84% of cases [12]; and that fewer than one in ten hospitalised patients are exposed to a clinically manifested drug interaction [4].
Only a minority of the alerts were deemed to merit additional action. Recommendations for management were often already attended to or not relevant in the specific case. As the alerts and recommendations continue to appear although the drug treatment is adequately managed, physicians may disregard them, thereby increasing the risk that important alerts are overlooked in a time-strained practice. To avoid information overload, one may hypothesize that the interaction alert system could benefit from increased integration with clinical data. For instance, although information sources may not be unambiguous [21], it could be valuable to incorporate dosing in the decision support system. In our study, for example, three out of nine omeprazole/(es)citalopram alerts did not require any action as a low dose was used. Further, two out of five D interaction alerts did not necessitate any action as at least one of the drugs was used PRN. On the other hand, our results illustrate that drugs used PRN may indeed be involved in drug interaction alerts where a related action is medically justified. Therefore, it may be problematic to reduce the alerts by simply applying a filter that makes the decision support system include only drugs prescribed regularly and in certain doses. However, one possibility could be to allow prescribers to temporarily disable interaction alerts already considered for a specific patient.
Alerts of the most serious category (D), where the recommendation is to avoid the drug combination, were shown in about one out of 30 patients. This prevalence of D interaction alerts is similar to the 3.8% reported in a previous study [22]. However, whereas the latter study was population-based, our study was restricted to patients 65 years or older with at least two drugs in their medication list. In addition, that study analysed drugs dispensed over a 4-month period, while we analysed the drugs actually included in a medication list after a planned consultation with a GP. Our study shows that less than half of the D alerts were medically warranted to act upon for the specific patient. Therefore, the prevalence of problematic D interactions in the general population may be considerably lower than indicated by prior research. The complexity of decision making is illustrated by our finding that one single action suggested in response to a D interaction concerned a simple switch from one drug to another within the same pharmacological subgroup. By contrast, all other actions due to D interactions required more complex clinical considerations.
Warfarin has been reported to be the drug most frequently seen in D interaction alerts [22]. Given the potentially severe consequences of interactions involving this substance, it is reassuring that, in our results, only one out of 26 C/D alerts including warfarin required medical action. In this case, the main issue was to check if an indication for the interacting agent (cholestyramine) persisted. In no case was the regular monitoring of INR problematic; such monitoring is well established in Sweden, with patients spending a high fraction of time in the therapeutic range [23]. Our results support findings in a previous study; integrating patient data, for instance laboratory parameters, may reduce alert severity in a non-negligible proportion of the interaction alerts, thereby increasing the alert specificity and decreasing the alert burden [24].
Almost half of the alerts were related to C interactions. Frequent C alerts concerned one drug affecting the absorption of another. Most of these alerts did not require any further medical action as the patient had already been informed to separate the intake. Almost all cases where the suggested action was a switch to another drug concerned substituting pantoprazole for omeprazole, which has been associated with a higher risk of drug interactions [25]. Interestingly, there were also some cases in which the indication for one or both interacting drugs was questioned and needed verification, an issue often encountered for PPIs [26]. These results may illustrate the importance of continuous medical reconsideration of drug treatment in relation to a patient’s evolving health status.
Alerts of B interactions were as common as C interactions. The most frequent B interaction alert concerned calcium and omeprazole, a combination that may impair the absorption of calcium and thereby contribute to an increased risk of fractures [27]. None of the B interactions was considered sufficient to justify any further medical action. This may not be surprising as B interactions are defined as having a clinical significance that either is “unknown” or “varies”.
According to Swedish regulations, each unit bag within a multi-dose drug dispensing system must be screened for drug interactions by a supervisory pharmacist [28], using the same drug interaction database (Janusmed) that automatically provides alerts for the prescribing physician. Despite this extra monitoring, the present study shows that one in eight interaction alerts for patients using this system could trigger a medical action. The fact that patients with multi-dose drug dispensing use many drugs may contribute to these findings. This system has indeed been associated with a rising number of drugs in the medication list [29], thereby increasing the potential for drug interactions [30]. Be that as it may, our results suggest that, despite control by the pharmacist, physicians need to pay attention to interaction alerts also among patients with multi-dose drug dispensing.
Our finding that only one in eleven interaction alerts seems to warrant further medical action for a specific patient suggests a need for caution in the interpretation of studies where quality of prescribing is equated with the surrogate measure “interaction alerts in a drug interaction database”. Including such alerts as measures in core outcome sets for the evaluation of interventions to improve prescribing practices, as recently proposed [13], could therefore be questioned.
Strengths and limitations
To the best of our knowledge, this is the first study to provide information on additional medically justified actions in response to drug interaction alerts from a computerised decision support system among older patients in primary care. An important strength of the study is the comprehensive drug treatment assessments performed, first independently and then in consensus, by two physicians specialised in clinical pharmacology and/or family medicine. This approach ensures that the results are relevant from a medical perspective. Nevertheless, the weak inter-rater agreement illustrates the complexity of pharmacotherapeutic assessments, as recently discussed [31]. Another strength is that warranted alert-related actions are described in detail.
As we included consecutive patients, from one urban and one rural primary health care centre staffed by more than 20 physicians at different career stages [15], the results are likely to be acceptably generalisable for older patents in primary care. However, differing prescribing practices within and between countries may have implications for the external validity. Nevertheless, frequent interaction alerts involving, for instance, metal ions potentially interacting with absorption and drug combinations resulting in an increased risk of bleeding, are in agreement with those previously reported [22]. Another aspect worth mentioning is that there are several available information resources for drug interactions, and these have been reported not to be entirely consistent [32,33,34].
It may be regarded as a limitation that the assessors were restricted to the information available in the primary care medical records; although this included hospital discharge summaries [15], all relevant information may not have been available. Therefore, there may be undocumented reasons for the prescribing physician not to act on the unaddressed alerts. Another limitation is that Janusmed, in accordance with other established decision support sources for potentially problematic drug interactions like Lexicomp, Micromedex, and Stockley’s Drug Interactions, provides information only for pairs of drugs, i.e. not for all drugs combined [35,36,37]. Indeed, a significant proportion of drug interaction queries to a drug information centre, where the entire medication list for a specific patient is usually considered, yielded advice for clinical action although no Janusmed alert was triggered [38]. Interestingly, a Swedish decision support system to guide clinicians regarding combined effects of multiple medicines is currently under development [39]. An additional limitation may be that Janusmed primarily covers pharmacokinetic interactions and therefore pharmacodynamic interactions may be underrepresented among the alerts. In the present study, however, an underlying pharmacodynamic mechanism was described in 81 (44%) alerted drug-drug pairs (data not shown). Finally, it must be stressed that this study does not evaluate the value of decision support regarding potential drug interactions at the initiation of drug treatment. Indeed, as we did not evaluate the drug treatment longitudinally, no detailed information regarding the prescribing physician’s management of interaction alerts could be provided.
Conclusion
This study shows that, using a computerised decision support system for drug interactions, alerts can be expected to be presented in more than every other older patient with two or more drugs in their medication list. Most of the alerts were already being addressed in health care, for instance by the monitoring of clinical or laboratory parameters, or were not relevant in the clinical setting. In about one in ten alerts, however, it may be appropriate to take further action, although, for unknown reasons, such steps were not taken. The underlying reasons for these alerts remaining unaddressed could be worth further investigation. As a minority of the alerts warrant medical action, interaction alerts seem to be of questionable value as indicators of problematic prescribing.
Data availability
The datasets generated and analysed during the current study are not publicly available owing to Swedish data protection laws. The data can be shared with authorised persons after approved application from the Swedish Ethical Review Authority (https://etikprovningsmyndigheten.se).
Code availability
Not applicable.
References
Zhang N, Sundquist J, Sundquist K, Ji J (2020) An increasing trend in the prevalence of polypharmacy in Sweden: a nationwide register-based study. Front Pharmacol 11:326. https://doi.org/10.3389/fphar.2020.00326
Dechanont S, Maphanta S, Butthum B, Kongkaew C (2014) Hospital admissions/visits associated with drug-drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 23:489–497. https://doi.org/10.1002/pds.3592
Zheng W, Richardson L, Li L, Day R, Westbrook J, Baysari M (2018) Drug-drug interactions and their harmful effects in hospitalised patients: a systematic review and meta-analysis. Eur J Clin Pharmacol 74:15–27. https://doi.org/10.1007/s00228-017-2357-5
Gonzaga de Andrade Santos TN, da Cruz M, Macieira G, Cardoso Sodré Alves BM, Onozato T, Cunha Cardoso G, Ferreira Nascimento MT, Saquete Martins-Filho PR, Pereira de Lyra D, Jr. Oliveira Filho AD (2020) Prevalence of clinically manifested drug interactions in hospitalized patients: A systematic review and meta-analysis. PLoS One 15:e0235353. https://doi.org/10.1371/journal.pone.0235353
Patel R, Beckett R (2016) Evalation of resources for analyzing drug interactions. J Med Libr Assoc 104:290–295
Roblek T, Vaupotic T, Mrhar A, Lainscak M (2015) Drug-drug interaction software in clinical practice: a systematic review. Eur J Clin Pharmacol 71:131–142. https://doi.org/10.1007/s00228-014-1786-7
Böttiger Y, Laine K, Andersson ML, Korhonen T, Molin B, Ovesjö ML et al (2009) SFINX-a drug-drug interaction database designed for clinical decision support systems. Eur J Clin Pharmacol 65: 627-633. https://doi.org/10.1007/s00228-008-0612-5
Fung KW, Kapusnik-Uner J, Cunningham J, Higby-Baker S, Bodenreider O (2017) Comparison of three commercial knowledge bases for detection of drug-drug interactions in clinical decision support. J Am Med Inform Assoc 24:806–812. https://doi.org/10.1093/jamia/ocx010
McEvoy DS, Sittig DF, Hickman TT, Aaron S, Ai A, Amato M, Bauer DW, Fraser GM, Harper J, Kennemer A, Krall MA, Lehmann CU, Malhotra S, Murphy DR, O’Kelley B, Samal L, Schreiber R, Singh H, Thomas EJ, Vartian CV, Westmorland J, McCoy AB, Wright A (2017) Variation in high-priority drug-drug interaction alerts across institutions and electronic health records. J Am Med Inform Assoc 24:331–338. https://doi.org/10.1093/jamia/ocw114
Andersson ML, Böttiger Y, Bastholm-Rahmner P, Ovesjö ML, Vég A, Eiermann B (2015) Evaluation of usage patterns and user perception of the drug-drug interaction database SFINX. Int J Med Inform 84:327–333. https://doi.org/10.1016/j.ijmedinf.2015.01.013
Peterson JF, Bates DW (2001) Preventable medication errors: identifying and eliminating serious drug interactions. J Am Pharm Assoc (Wash) 41:159–160. https://doi.org/10.1016/s1086-5802(16)31243-8
Poly TN, Islam MM, Yang HC, Li YJ (2020) Appropriateness of overridden alerts in computerized physician order entry: systematic review. JMIR Med Inform 8:e15653. https://doi.org/10.2196/15653
Beuscart JB, Knol W, Cullinan S, Schneider C, Dalleur O, Boland B, Thevelin S, Jansen PAF, O’Mahony D, Rodondi N, Spinewine A (2018) International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med 16:21. https://doi.org/10.1186/s12916-018-1007-9
Rankin A, Cadogan CA, In Ryan C, Clyne B, Smith SM, Hughes CM (2018) Core outcome set for trials aimed at improving the appropriateness of polypharmacy in older people in primary care. J Am Geriatr Soc 66:1206–1212. https://doi.org/10.1111/jgs.15245
Parodi López N, Svensson SA, Wallerstedt SM (2021) Association between recorded medication reviews in primary care and adequate drug treatment management - a cross-sectional study. Scand J Prim Health Care 39:419-428. https://doi.org/10.1080/02813432.2021.1973239
Andersson ML, Böttiger Y, Lindh JD, Wettermark B, Eiermann B et al (2013) Impact of the drug-drug interaction database SFINX on prevalence of potentially serious drug-drug interactions in primary health care. Eur J Clin Pharmacol 69:565–571
Stockholm Regional Council, the Health and Medical Care Administration. Janusmed interactions. In: ed. Avaible at: http://www.janusinfo.se. Accessed January 2020.
O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P (2015) STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 44:213–218. https://doi.org/10.1093/ageing/afu145
Fastbom J, Johnell K (2015) National indicators for quality of drug therapy in older persons: the Swedish experience from the first 10 years. Drugs Aging 32:189–199. https://doi.org/10.1007/s40266-015-0242-4
Sjöberg C, Ohlsson H, Wallerstedt SM (2012) Association between multi-dose drug dispensing and drug treatment changes. Eur J Clin Pharmacol 68:1095–1101. https://doi.org/10.1007/s00228-012-1230-9
Seidling HM, Al Barmawi A, Kaltschmidt J, Bertsche T, Pruszydlo MG, Haefeli WE (2007) Detection and prevention of prescriptions with excessive doses in electronic prescribing systems. Eur J Clin Pharmacol 63:1185–1192. https://doi.org/10.1007/s00228-007-0370-9
Holm J, Eiermann B, Eliasson E, Mannheimer B (2014) A limited number of prescribed drugs account for a great majority of drug-drug interactions. Eur J Clin Pharmacol 70:1375–1383
Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, Pais P, Dans A, Eikelboom J, Oldgren J, Pogue J, Reilly PA, Yang S, Connolly SJ (2010) Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 376:975–983. https://doi.org/10.1016/s0140-6736(10)61194-4
Seidling HM, Klein U, Schaier M, Czock D, Theile D, Pruszydlo MG, Kaltschmidt J, Mikus G, Haefeli WE (2014) What, if all alerts were specific - estimating the potential impact on drug interaction alert burden. Int J Med Inform 83:285–291. https://doi.org/10.1016/j.ijmedinf.2013.12.006
Wedemeyer RS, Blume H (2014) Pharmacokinetic drug interaction profiles of proton pump inhibitors: an update. Drug Saf 37:201–211. https://doi.org/10.1007/s40264-014-0144-0
Wallerstedt SM, Fastbom J, Linke J, Vitols S (2017) Long-term use of proton pump inhibitors and prevalence of disease- and drug-related reasons for gastroprotection-a cross-sectional population-based study. Pharmacoepidemiol Drug Saf 26:9–16. https://doi.org/10.1002/pds.4135
O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ (2005) Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med 118:778–781. https://doi.org/10.1016/j.amjmed.2005.02.007
Governmental investigation (2018) Review of multi-dose drug dispensing, extempore, trial drugs et cetera. [Statens offentliga utredningar. Översyn av maskinell dos, extempore, prövningsläkemedel m.m.] SOU 2018:53. In: ed. https://www.regeringen.se/rattsliga-dokument/statens-offentliga-utredningar/2018/06/sou-201853/. Accessed 15 June 2021
Wallerstedt SM, Fastbom J, Johnell K, Sjöberg C, Landahl S, Sundström A (2013) Drug treatment in older people before and after the transition to a multi-dose drug dispensing system–a longitudinal analysis. PLoS One 8:e67088. https://doi.org/10.1371/journal.pone.0067088
Josendal AV, Bergmo TS, Granas AG (2020) Potentially inappropriate prescribing to older patients receiving multidose drug dispensing. BMC Geriatr 20:272. https://doi.org/10.1186/s12877-020-01665-x
Lönnbro J, Holmqvist L, Persson E, Thysell P, Åberg ND, Wallerstedt SM (2021) Inter-rater reliability of assessments regarding the quality of drug treatment, and drug-related hospital admissions. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.14790
Beckett RD, Martin JR, Stump CD, Dyer MA (2020) Evaluation of drug information resources for interactions between therapeutic drugs and drugs of abuse. J Med Libr Assoc 108:584–590. https://doi.org/10.5195/jmla.2020.969
Castaldelli-Maia JM, Hofmann C, Chagas ACP, Liprandi AS, Alcocer A, Andrade LH, Wielgosz A (2020) Major cardiac-psychiatric drug-drug interactions: a systematic review of the consistency of drug databases. Cardiovasc Drugs Ther. https://doi.org/10.1007/s10557-020-06979-x
Patel RI, Beckett RD (2016) Evaluation of resources for analyzing drug interactions. J Med Libr Assoc 104:290–295. https://doi.org/10.3163/1536-5050.104.4.007
IBM Micromedex http://www.micromedexsolutions.com Greenwood Village, Colorado, USA.IBM Watson Health. Accessed 2021
Stockley´s Drug Interactions. https://about.medicinescomplete.com/publication/stockleys-drug-interactions. Pharmaceutical Press. Accessed 2021
Lexicomp interactions Ld. https://about.medicinescomplete.com/publication/stockleys-drug-interactions/. Waltham (MA): UpToDate. Accessed 2021
Tukukino C, Wallerstedt SM (2019) Drug information centre queries and responses about drug interactions over 10 years-a descriptive analysis. Basic Clin Pharmacol Toxicol 126:65–74. https://doi.org/10.1111/bcpt.13294
Böttiger Y, Laine K, Korhonen T, Lahdesmaki J, Shemeikka T, Julander M, Edlert M, Andersson ML (2018) Development and pilot testing of PHARAO-a decision support system for pharmacological risk assessment in the elderly. Eur J Clin Pharmacol 74:365–371. https://doi.org/10.1007/s00228-017-2391-3
Funding
Open access funding provided by University of Gothenburg. This study was funded by grants from the Swedish Research Council (521–2013-2639) and the Swedish state under the ALF agreement between the Swedish government and the county councils (ALFGBG-716941).
Author information
Authors and Affiliations
Contributions
Carina Tukukino and Susanna M Wallerstedt conceived and designed the study. Naldy Parodi López and Staffan A Svensson performed the pharmacotherapy assessments. Carina Tukukino extracted the interaction alerts. Carina Tukukino and Naldy Parodi López prepared the database used for analysis. Carina Tukukino and Susanna M Wallerstedt drafted the manuscript and all authors revised it for intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Regional Ethical Review Board in Gothenburg, Sweden (DRN: 1046–15).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1
Appendix 1
Guiding sentences for the assessment of whether an action related to the drug treatment, including the drug interactions alerted for, was medically justified at the individual level.
1 | I would not change anything in the patient’s drug treatment |
2 | I would reconsider the drug treatment in the long term but would do nothing during the current visit; it could be reassessed at the next regular consultation |
3 | I would take some action to be able to make a decision regarding the drug treatment, for instance order a laboratory test, get more information about the patient or schedule an extra visit, before the next regular consultation |
4 | I would change the drug treatment at the index visit |
Sentences (3) and (4) were collapsed into one category where some actions were considered medically justified prior to the next regular visit, whereas sentences (1) and (2) reflected that no action was required.
Taken from: Parodi López N, Svensson SA, Wallerstedt SM. Association between recorded medication reviews in primary care and adequate drug treatment management – a cross-sectional study. Scand J Prim Health Care. 2021; 39:419-428. https://doi.org/10.1080/02813432.2021.1973239.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tukukino, C., Parodi López, N., Svensson, S.A. et al. Drug interaction alerts in older primary care patients, and related medically justified actions. Eur J Clin Pharmacol 78, 1115–1126 (2022). https://doi.org/10.1007/s00228-022-03292-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-022-03292-4