Abstract
Purpose
Study drug discontinuation is commonplace in clinical trials of older populations. Little is known about why older participants discontinue the study drug. This qualitative study aimed to understand factors contributing to permanent study drug discontinuation among participants aged ≥ 70 years within an ongoing primary prevention trial of statins by exploring their experiences and perceptions.
Methods
Trial participants who had permanently discontinued the study drug within 2 years of randomisation were purposively sampled by age (< 75 and ≥ 75 years) and sex to participate in semi-structured phone interviews between March 2019 and February 2020. Interviews were audio-recorded, transcribed and analysed thematically.
Results
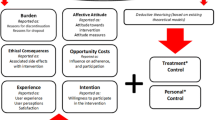
Thirty participants were interviewed (21 females; mean age, 77 years), and three themes were identified from the data. Perceived adverse events (AEs) and their effect on daily living (mobility, functional capacity, quality of life) were identified as the major factors leading to the participants permanently discontinuing their study drug, despite an ambiguity about the cause of the AE. For some, concurrent challenging life circumstances further lowered their tolerance to perceived AEs thus making discontinuation more likely. A few discontinuations were attributed to other factors (e.g. GP advice, unrelated illness).
Conclusion
Among healthy older participants enrolled in a statin trial, perceived AEs and their related impact were key factors contributing to the permanent study drug discontinuation. Addressing anticipated participant-reported AEs and their concerns about drug-related side effects at trial entry, as well as offering timely medical assistance and support when AEs occur, may be useful to reduce drug discontinuation rates.
Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Yazdanyar A, Newman AB (2009) The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clin Geriatr Med 25(4):563–577 vii
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy J, Michos ED, Miedema MD, Muñoz D, Smith SC Jr, Virani SS, Williams KA Sr, Yeboah J, Ziaeian B (2019) 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 140(11):e596–e646
Hariton E, Locascio JJ (2018) Randomised controlled trials - the gold standard for effectiveness research: study design: randomised controlled trials. BJOG. 125(13):1716
Benetos A, Petrovic M, Strandberg T (2019) Hypertension management in older and frail older patients. Circ Res 124(7):1045–1060
Zweben A, Fucito LM, O'Malley SS (2009) Effective strategies for maintaining research participation in clinical trials. Drug Inf J 43(4):459–467
Kesten S, Plautz M, Piquette CA, Habib MP, Niewoehner DE (2007) Premature discontinuation of patients: a potential bias in COPD clinical trials. Eur Respir J 30(5):898–906
Shiovitz TM, Bain EE, McCann DJ, Skolnick P, Laughren T, Hanina A et al (2016) Mitigating the effects of nonadherence in clinical trials. J Clin Pharmacol 56(9):1151–1164
Smith DL (2012) Patient nonadherence in clinical trials: could there be a link to postmarketing patient safety? Drug Inf J 46(1):27–34
Topinkova E (2008) Aging, disability and frailty. Ann Nutr Metab 52(Suppl 1):6–11
Hassanzadeh A, Heidari Z, Feizi A, Hassanzadeh Keshteli A, Roohafza H, Afshar H et al (2017) Association of stressful life events with psychological problems: a large-scale community-based study using grouped outcomes latent factor regression with latent predictors. Comput Math Methods Med 2017:3457103
Banach M, Rizzo M, Toth PP, Farnier M, Davidson MH, Al-Rasadi K et al (2015) Statin intolerance - an attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch Med Sci 11(1):1–23
Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B (2012) Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 380(9836):37–43
Navar AM, Roe MT, White JA, Cannon CP, Lokhnygina Y, Newby LK, Giugliano RP, Tershakovec AM, Braunwald E, Califf RM, Blazing MA (2019) Medication discontinuation in the IMPROVE-IT trial. Circ Cardiovasc Qual Outcomes 12(1):e005041
Skea ZC, Newlands R, Gillies K (2019) Exploring non-retention in clinical trials: a meta-ethnographic synthesis of studies reporting participant reasons for drop out. BMJ Open 9(6):e021959
Zoungas S, Curtis A, Tonkin A, McNeil J (2014) Statins in the elderly: an answered question? Curr Opin Cardiol 29(4):372–380
Patton MQ (2002) Qualitative research and evaluation methods. Sage Publications, Thousand Oaks
Gale NK, Heath G, Cameron E, Rashid S, Redwood S (2013) Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 13:117
Adamson J, Hewitt CE, Torgerson DJ (2015) Producing better evidence on how to improve randomised controlled trials. BMJ. 351:h4923
Schulz KF, Grimes DA (2002) Sample size slippages in randomised trials: exclusions and the lost and wayward. Lancet. 359(9308):781–785
Gardette V, Coley N, Toulza O, Andrieu S (2007) Attrition in geriatric research: how important is it and how should it be dealt with? J Nutr Health Aging 11(3):265–271
Macias FM, Ramsay RE, Rowan AJ (2007) Recruitment and retention in clinical trials of the elderly. Int Rev Neurobiol 81:265–272
Saib A, Sabbah L, Perdrix L, Blanchard D, Danchin N, Puymirat E (2013) Evaluation of the impact of the recent controversy over statins in France: the EVANS study. Arch Cardiovasc Dis 106(10):511–516
O'Cathain A, Thomas KJ, Drabble SJ, Rudolph A, Goode J, Hewison J (2014) Maximising the value of combining qualitative research and randomised controlled trials in health research: the QUAlitative Research in Trials (QUART) study--a mixed methods study. Health Technol Assess 18(38):1–197 v-vi
Fried TR, Tinetti ME, Towle V, O'Leary JR, Iannone L (2011) Effects of benefits and harms on older persons' willingness to take medication for primary cardiovascular prevention. Arch Intern Med 171(10):923–928
Beeson D (1997) Nuance, complexity, and context: qualitative methods in genetic counseling research. J Genet Couns 6(1):21–43
Rosenbaum D, Dallongeville J, Sabouret P, Bruckert E (2013) Discontinuation of statin therapy due to muscular side effects: a survey in real life. Nutr Metab Cardiovasc Dis 23(9):871–875
Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, de Backer G, Krauss RM, Laufs U, Santos RD, Hegele RA, Hovingh GK, Leiter LA, Mach F, März W, Newman CB, Wiklund O, Jacobson TA, Catapano AL, Chapman MJ, Ginsberg HN, European Atherosclerosis Society Consensus Panel, Stroes E, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, Roden M, Stein E, Tokgözoğlu L, Nordestgaard BG, Bruckert E, Krauss RM, Laufs U, Santos RD, März W, Newman CB, John Chapman M, Ginsberg HN, John Chapman M, Ginsberg HN, de Backer G, Catapano AL, Hegele RA, Kees Hovingh G, Jacobson TA, Leiter L, Mach F, Wiklund O (2015) Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J 36(17):1012–1022
Eborall HC, Stewart MC, Cunningham-Burley S, Price JF, Fowkes FG (2011) Accrual and drop out in a primary prevention randomised controlled trial: qualitative study. Trials. 12:7
Lockery JE, Collyer TA, Abhayaratna WP, Fitzgerald SM, McNeil JJ, Nelson MR et al (2019) Recruiting general practice patients for large clinical trials: lessons from the Aspirin in Reducing Events in the Elderly (ASPREE) study. Med J Aust 210(4):168–173
Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, Frankel S, Neal D, Hamdy F (2002) Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ. 325(7367):766–770
Chew-Graham CA, Shepherd T, Burroughs H, Dixon K, Kessler D (2018) The value of an embedded qualitative study in a trial of a second antidepressant for people who had not responded to one antidepressant: understanding the perspectives of patients and general practitioners. BMC Fam Pract 19(1):197
Clark L, Ronaldson S, Dyson L, Hewitt C, Torgerson D, Adamson J (2015) Electronic prompts significantly increase response rates to postal questionnaires: a randomized trial within a randomized trial and meta-analysis. J Clin Epidemiol 68(12):1446–1450
Acknowledgements
The authors would like to thank the STAREE (Statins in Reducing Events in the Elderly) participants in this study who gave their precious time for interviews, and the STAREE Investigator Group for their support. Special thanks also go to Simona Markusevska and Naomi Szwarcbard who assisted in participant recruitment for this study.
Funding
STAREE has been awarded project grants from the NHMRC (Australia) (APP1068146 and APP1161503) and from a Stroke Prevention Grant from the Heart Foundation (#101663). There is no funding that specifically supports this qualitative research.
Author information
Authors and Affiliations
Consortia
Contributions
Study concept and design: ZZ, KJ, AC, AK, SZ, MB, MN. Acquisition, analysis or interpretation of data: ZZ, KJ, AK. Drafting of the manuscript: ZZ. Critical revision of the manuscript for important intellectual content: ZZ, KJ, AC, AK, SZ, MB, MN. Administrative, technical or material support: AK, SZ. Study supervision: MN.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Monash University Human Research Ethics Committee (15334) and Tasmanian Health and Medical Human Research Ethics Committee (H0017572).
Consent to participate
Verbal informed consent was obtained prior to the interview.
Consent to publish
Not applicable. No identifying information is included in this article.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Adverse events along with their impacts on daily living were identified as the major factors leading to older participants permanently discontinuing their study drug.
• Difficult life circumstances may increase the chance of study drug discontinuation among older trial participants.
• Proposed strategies may help prevent study drug discontinuation in older trial participants.
Rights and permissions
About this article
Cite this article
Zhou, Z., Jose, K., Curtis, A.J. et al. Older participant perspectives on permanent study drug discontinuation in an ongoing primary prevention trial of statins. Eur J Clin Pharmacol 77, 841–847 (2021). https://doi.org/10.1007/s00228-020-03073-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-020-03073-x