Abstract
Purpose
The primary aim was to examine and compare the increased risk of incident diabetes associated with second-generation antipsychotics (SGAs) and first-generation antipsychotics (FGAs), with and without adjusting for potential confounding factors. The secondary aim was to recalculate the relative risks of diabetes onset using a semi-symmetric bidirectional case-crossover (SSBC) design to adjust for time-trend bias.
Method
Prescription records (2005–2015) of antipsychotics were sourced from New Zealand Pharmaceutical Collections. The first-time diabetes diagnosis was extracted from the National Minimal Dataset. Relative risks (RRs) of diabetes onset were calculated using conditional logistic regression. Time-trend bias was corrected by recalculating the RR using a SSBC design.
Results
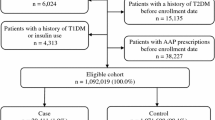
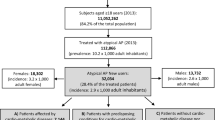
Among 645 individuals, the risk of diabetes onset is higher in SGA users (ARR = 8.72, 95% CI = [5.57, 13.67]) compared to FGA users (ARR = 5.68, 95% CI = [3.43, 9.39]). The increased risk of diabetes onset associated with quetiapine is higher (ARR = 7.47, 95% CI = [4.10, 13.62]), compared to haloperidol (ARR = 5.05, 95% CI = [2.91, 8.75]). However, the increased risk of diabetes onset associated with olanzapine (ARR = 2.27, 95% CI = [0.86, 5.98]) is insignificant after adjusting for concomitant use of effect modifiers and other antipsychotic drugs.
Conclusion
The results support that the magnitude of the risk of diabetes is higher with SGA use compared with FGA use, and the risk is higher when co-prescribed. Confounding by indication and time-varying confounders such as body mass index could bias the risk of onset of diabetes. Marginal structural models could provide more precise estimates of the risk of onset of diabetes following exposure to antipsychotics.


Similar content being viewed by others
References
Stahl SM (1999) Selecting an atypical antipsychotic by combining clinical experience with guidelines from clinical trials. The Journal of clinical psychiatry 60(Suppl 10):31–41
Jakovljevic M (2009) New generation vs. first generation antipsychotics debate: pragmatic clinical trials and practice-based evidence. Psychiatr Danub 21(4):446–452
Lambert M, Schimmelmann BG, Schacht A, Suarez D, Haro JM, Novick D, Wagner T, Wehmeier PM, Huber CG, Hundemer HP, Dittmann RW, Naber D (2011) Differential 3-year effects of first- versus second-generation antipsychotics on subjective well-being in schizophrenia using marginal structural models. J Clin Psychopharmacol 31(2):226–230. doi:10.1097/JCP.0b013e3182114d21
Buse JB, Cavazzoni P, Hornbuckle K, Hutchins D, Breier A, Jovanovic L (2003) A retrospective cohort study of diabetes mellitus and antipsychotic treatment in the United States. J Clin Epidemiol 56(2):164–170
Caro JJ, Ward A, Levinton C, Robinson K (2002) The risk of diabetes during olanzapine use compared with risperidone use: a retrospective database analysis. The Journal of clinical psychiatry 63(12):1135–1139
Feldman PD, Hay LK, Deberdt W, Kennedy JS, Hutchins DS, Hay DP, Hardy TA, Hoffmann VP, Hornbuckle K, Breier A (2004) Retrospective cohort study of diabetes mellitus and antipsychotic treatment in a geriatric population in the United States. J Am Med Dir Assoc 5(1):38–46
Fuller MA, Shermock KM, Secic M, Grogg AL (2003) Comparative study of the development of diabetes mellitus in patients taking risperidone and olanzapine. Pharmacotherapy 23(8):1037–1043
Ulcickas Yood M, Delorenze GN, Quesenberry CP Jr, Oliveria SA, Tsai AL, Kim E, Cziraky MJ, McQuade RD, Newcomer JW, L'Italien GJ (2011) Association between second-generation antipsychotics and newly diagnosed treated diabetes mellitus: does the effect differ by dose? BMC psychiatry 11:197. doi:10.1186/1471-244x-11-197
Yood MU, DeLorenze G, Quesenberry CP Jr, Oliveria SA, Tsai AL, Willey VJ, McQuade R, Newcomer J, L’Italien G (2009) The incidence of diabetes in atypical antipsychotic users differs according to agent—results from a multisite epidemiologic study. Pharmacoepidemiol Drug Saf 18(9):791–799. doi:10.1002/pds.1781
Montgomery JH, Byerly M, Carmody T, Li B, Miller DR, Varghese F, Holland R (2004) An analysis of the effect of funding source in randomized clinical trials of second generation antipsychotics for the treatment of schizophrenia. Control Clin Trials 25(6):598–612. doi:10.1016/j.cct.2004.09.002
Peluso MJ, Lewis SW, Barnes TR, Jones PB (2012) Extrapyramidal motor side-effects of first- and second-generation antipsychotic drugs. The British journal of psychiatry : the journal of mental science 200(5):387–392. doi:10.1192/bjp.bp.111.101485
Delaney JA, Suissa S (2009) The case-crossover study design in pharmacoepidemiology. Stat Methods Med Res 18(1):53–65. doi:10.1177/0962280208092346
Maclure M (1991) The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 133(2):144–153
Chang CH, Chen HC, Lin JW, Kuo CW, Shau WY, Lai MS (2011) Risk of hospitalization for upper gastrointestinal adverse events associated with nonsteroidal anti-inflammatory drugs: a nationwide case-crossover study in Taiwan. Pharmacoepidemiol Drug Saf 20(7):763–771. doi:10.1002/pds.2140
Schulte PF, Bocxe JT, Doodeman HJ, van Haelst IM, Cohen D (2016) Risk of new-onset diabetes after long-term treatment with clozapine in comparison to other antipsychotics in patients with schizophrenia. J Clin Psychopharmacol 36(2):115–119. doi:10.1097/jcp.0000000000000465
Rubin DM, Kreider AR, Matone M, Huang YS, Feudtner C, Ross ME, Localio AR (2015) Risk for incident diabetes mellitus following initiation of second-generation antipsychotics among Medicaid-enrolled youths. JAMA Pediatr 169(4):e150285. doi:10.1001/jamapediatrics.2015.0285
Tandon R (2002) Safety and tolerability: how do newer generation “atypical” antipsychotics compare? The Psychiatric quarterly 73(4):297–311
Miron IC, Baroana VC, Popescu F, Ionica F (2014) Pharmacological mechanisms underlying the association of antipsychotics with metabolic disorders. Current health sciences journal 40(1):12–17. doi:10.12865/chsj.40.01.02
Nielsen J, Skadhede S, Correll CU (2010) Antipsychotics associated with the development of type 2 diabetes in antipsychotic-naive schizophrenia patients. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 35(9):1997–2004. doi:10.1038/npp.2010.78
2004) Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 27(2):596–601
Baker RA, Pikalov A, Tran QV, Kremenets T, Arani RB, Doraiswamy PM (2009) Atypical antipsychotic drugs and diabetes mellitus in the US Food and Drug Administration adverse event database: a systematic Bayesian signal detection analysis. Psychopharmacol Bull 42(1):11–31
DuMouchel W, Fram D, Yang X, Mahmoud RA, Grogg AL, Engelhart L, Ramaswamy K (2008) Antipsychotics, glycemic disorders, and life-threatening diabetic events: a Bayesian data-mining analysis of the FDA adverse event reporting system (1968-2004). Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists 20(1):21–31. doi:10.1080/10401230701844612
Navidi W, Weinhandl E (2002) Risk set sampling for case-crossover designs. Epidemiology 13(1):100–105
Acknowledgments
The authors would like to thank the Analytical Services, Ministry of Health of New Zealand for providing the datasets and the Research in Pharmacoepidemiology (RiPE) group, School of Pharmacy, University of Otago for providing clinical data management and support. All patient data used in this study are de-identified.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nishtala, P.S., Chyou, Ty. Real-world risk of diabetes with antipsychotic use in older New Zealanders: a case-crossover study. Eur J Clin Pharmacol 73, 233–239 (2017). https://doi.org/10.1007/s00228-016-2158-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2158-2