Abstract
Purpose
The purpose of this study was to investigate changes in utilisation of antiepileptic drugs (AEDs) in epilepsy and non-epilepsy disorders in Norway and furthermore to study the retention rates of the most commonly used AEDs in these indications in long-term use.
Methods
The data consisted of all prescriptions of AEDs from Norwegian pharmacies in the Norwegian Prescription Database (NorPD) (2004–2012). Variables included anonymous data regarding age, gender, diagnosis specific reimbursement codes and utilisation of AEDs.
Results
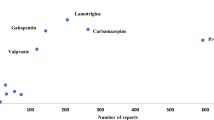
In recent years (2008–2012), the utilisation of AEDs in non-epilepsy disorders accounted for 45–53 % of the total use. In epilepsy, the most commonly used AED was lamotrigine, followed by levetiracetam, carbamazepine and valproate. Lamotrigine was also the predominant AED used in psychiatry, while pregabalin and gabapentin were mostly used in neuropathic pain. In migraine, topiramate predominated but accounted for <1 % of the total utilisation of AEDs. The majority of prescriptions were by general practitioners and only 20 % by specialists. Regardless of indication, newer AEDs had higher retention rates (34–48 %) and were used for a longer period before discontinuation.
Conclusions
The use of AEDs in non-epilepsy disorders is increasing and accounted for 53 % in 2012. Newer AEDs were predominantly used and demonstrated higher retention rates than older AEDs in all indications. This nationwide study demonstrates an increased exposure to AEDs in new patient groups, and details in prescription patterns and clinical and safety considerations should be closely monitored. This contributes to long-term post-marketing data of AED and accordingly improved pharmacovigilance.



Similar content being viewed by others
References
Tsiropoulos I, Gichangi A, Andersen M, Bjerrum L, Gaist D, Hallas J (2006) Trends in utilization of antiepileptic drugs in Denmark. Acta Neurol Scand 113:405–411
Savica R, Beghi E, Mazzaglia G, Innocenti F, Brignoli O, Cricelli C, Caputi AP, Musolino R, Spina E, Trifirò G (2007) Prescribing patterns of antiepileptic drugs in Italy: a nationwide population-based study in the years 2000-2005. Eur J Neurol Off J Eur Fed Neurol Soc 14:1317–1321
Johannessen Landmark C, Larsson PG, Rytter E, Johannessen SI (2009) Antiepileptic drugs in epilepsy and other disorders—a population-based study of prescriptions. Epilepsy Res 87:31–39
Landmark CJ, Fossmark H, Larsson PG, Rytter E, Johannessen SI (2011) Prescription patterns of antiepileptic drugs in patients with epilepsy in a nation-wide population. Epilepsy Res 95:51–59
Oteri A, Trifiro G, Gagliostro MS, Tari DU, Moretti S, Bramanti P, Spina E, Caputi AP, Arcoraci V (2010) Prescribing pattern of anti-epileptic drugs in an Italian setting of elderly outpatients: a population-based study during 2004-07. Br J Clin Pharmacol 70:514–522
Italiano D, Capuano A, Alibrandi A, Ferrara R, Cannata A, Trifiro G, Sultana J, Ferrajolo C, Tari M, Tari DU, Perrotta M, Pagliaro C, Rafaniello C, Spina E, Arcoraci V (2015) Indications of newer and older anti-epileptic drug use: findings from a southern Italian general practice setting from 2005-2011. Br J Clin Pharmacol 79:1010–1019
Johannessen Landmark C, Johannessen SI, Tomson T (2012) Host factors affecting antiepileptic drug delivery-pharmacokinetic variability. Adv Drug Deliv Rev 64:896–910
Johannessen Landmark C (2008) Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs 22:27–47
Landmark CJ, Johannessen SI (2012) Safety aspects of antiepileptic drugs—focus on pharmacovigilance. Pharmacoepidemiol Drug Saf 21:11–20
Takahashi Y, Nishida Y, Asai S (2012) Utilization of health care databases for pharmacoepidemiology. Eur J Clin Pharmacol 68:123–129
Wise L (2011) Risks and benefits of (pharmaco)epidemiology. Ther Adv Drug Saf 2:95–102
Hamer HM, Dodel R, Strzelczyk A, Balzer-Geldsetzer M, Reese JP, Schoffski O, Graf W, Schwab S, Knake S, Oertel WH, Rosenow F, Kostev K (2012) Prevalence, utilization, and costs of antiepileptic drugs for epilepsy in Germany—a nationwide population-based study in children and adults. J Neurol 259:2376–2384
WHO. (2002) The importance of pharmacovigilance. Safety Monitoring of Medicinal Products. World Health Organization 2002.
WHO Collaboration Center. Available at http://www.whocc.no.
The Norwegian Institute of Public Health, The National Prescription Database (NorPD). Available at: http://www.reseptregisteret.no.
Furu K (2008) Establishment of the nationwide Norwegian Prescription Database (NorPD)—new opportunities for research in pharmacoepidemiology in Norway. Nor Epidemiol 18:129–136
ICD-10 classification system. Available at: http://www.who.int/classifications/icd/en/.
ICPC-2 classification system. Available at: https://helsedirektoratet.no/helsefaglige-kodeverk/icpc-2-den-internasjonale-klassifikasjonen-for-primerhelsetjenesten. [Norwegian]
Statistics Norway. Available at: http://www.ssb.no.
The Norwegian Medicines Agency. Reimbursement status for pregabalin. Available at: http://www.legemiddelverket.no.
The Norwegian Medicines Agency. Reimbursement status for gabapentin. Available at: http://www.legemiddelverket.no.
Glauser T, Ben-Menachem E, Bourgeois B, Cnaan A, Guerreiro C, Kalviainen R, Mattson R, French JA, Perucca E, Tomson T (2013) Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 54:551–563
Karouni M, Arulthas S, Larsson PG, Rytter E, Johannessen SI, Landmark CJ (2010) Psychiatric comorbidity in patients with epilepsy: a population-based study. Eur J Clin Pharmacol 66:1151–1160
Connolly KR, Thase ME. The clinical management of bipolar disorder: a review of evidence-based guidelines. The Primary Care Companion for CNS Disorders 2011;13
Bramness JG, Groholt B, Engeland A, Furu K (2009) The use of lithium, valproate or lamotrigine for psychiatric conditions in children and adolescents in Norway 2004-2007—a prescription database study. J Affect Disord 117:208–211
Golden AS, Haut SR, Moshe SL (2006) Nonepileptic uses of antiepileptic drugs in children and adolescents. Pediatr Neurol 34:421–432
Citrome L (2009) Adjunctive lithium and anticonvulsants for the treatment of schizophrenia: what is the evidence? Expert Rev Neurother 9:55–71
Mason J, Pirmohamed M, Nunn T (2012) Off-label and unlicensed medicine use and adverse drug reactions in children: a narrative review of the literature. Eur J Clin Pharmacol 68:21–28
Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, Nurmikko T (2010) EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol Off J Eur Fed Neurol Soc 17:1113–1188
Perez C, Navarro A, Saldana MT, Masramon X, Rejas J (2010) Pregabalin and gabapentin in matched patients with peripheral neuropathic pain in routine medical practice in a primary care setting: findings from a cost-consequences analysis in a nested case-control study. Clin Ther 32:1357–1370
Landmark CJ, Larsson PG, Rytter E, Johannessen SI (2011) Clarification about pregabalin and the Norwegian Prescription Database. Tidsskr Nor Laegeforen 131:800–801 [Norwegian]
Caster O, Edwards IR, Noren GN, Lindquist M (2011) Earlier discovery of pregabalin’s dependence potential might have been possible. Eur J Clin Pharmacol 67:319–320
Gabapentin and pregabalin: abuse and addiction. Prescrire Int 2012; 21: 152–4.
Kruszewski SP, Paczynski RP, Kahn DA (2009) Gabapentin-induced delirium and dependence. J Psychiatr Pract 15:314–319
Gidal BE, Radulovic LL, Kruger S, Rutecki P, Pitterle M, Bockbrader HN (2000) Inter- and intra-subject variability in gabapentin absorption and absolute bioavailability. Epilepsy Res 40:123–127
Johannessen Landmark C, Beiske G, Baftiu A, Burns ML, Johannessen SI (2015) Experience from therapeutic drug monitoring and gender aspects of gabapentin and pregabalin in clinical practice. Seizure 28:88–91
Patsalos PN, Berry DJ, Bourgeois BF, Cloyd JC, Glauser TA, Johannessen SI, Leppik IE, Tomson T, Perucca E (2008) Antiepileptic drugs—best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 49:1239–1276
Tomson T, Landmark CJ, Battino D (2013) Antiepileptic drug treatment in pregnancy: changes in drug disposition and their clinical implications. Epilepsia 54:405–414
Baftiu A, Johannessen Landmark C, Nikaj V, Neslein IL, Johannessen SI, Perucca E (2015) Availability of antiepileptic drugs across Europe. Epilepsia 56:e191–e197
Saetre E, Perucca E, Isojarvi J, Gjerstad L (2007) An international multicenter randomized double-blind controlled trial of lamotrigine and sustained-release carbamazepine in the treatment of newly diagnosed epilepsy in the elderly. Epilepsia 48:1292–1302
Werhahn KJ, Trinka E, Dobesberger J, Unterberger I, Baum P, Deckert-Schmitz M, Kniess T, Schmitz B, Bernedo V, Ruckes C, Ehrlich A, Kramer G (2015) A randomized, double-blind comparison of antiepileptic drug treatment in the elderly with new-onset focal epilepsy. Epilepsia 56:450–459
Chung S, Wang N, Hank N (2007) Comparative retention rates and long-term tolerability of new antiepileptic drugs. Seizure 16:296–304
de Groot MC, Schuerch M, de Vries F, Hesse U, Oliva B, Gil M, Huerta C, Requena G, de Abajo F, Afonso AS, Souverein PC, Alvarez Y, Slattery J, Rottenkolber M, Schmiedl S, Van Dijk L, Schlienger RG, Reynolds R, Klungel OH (2014) Antiepileptic drug use in seven electronic health record databases in Europe: a methodologic comparison. Epilepsia 55:666–673
Giussani G, Canelli V, Bianchi E, Franchi C, Nobili A, Erba G, Beghi E (2016) A population-based study of active and drug-resistant epilepsies in Northern Italy. Epilepsy Behav: EnB 55:30–37
Wettermark B, Zoega H, Furu K, Korhonen M, Hallas J, Norgaard M, Almarsdottir A, Andersen M, Andersson Sundell K, Bergman U, Helin-Salmivaara A, Hoffmann M, Kieler H, Martikainen J, Mortensen M, Petzold M, Wallach-Kildemoes H, Wallin C, Sørensen H (2013) The Nordic prescription databases as a resource for pharmacoepidemiological research—a literature review. Pharmacoepidemiol Drug Saf 22:691–699
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Norwegian Institute of Public Health. The ethical considerations included that the data were anonymous with no patient identification, as each patient was given a running number in the data file (pseudonymous), and Statistics Norway provided security for protection of patient information.
Disclosure
The authors have no conflicts of interest or any financial disclosures, sponsors or grants regarding this manuscript. The results have not previously been presented.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00228-017-2252-0.
Rights and permissions
About this article
Cite this article
Baftiu, A., Johannessen Landmark, C., Rusten, I.R. et al. Changes in utilisation of antiepileptic drugs in epilepsy and non-epilepsy disorders—a pharmacoepidemiological study and clinical implications. Eur J Clin Pharmacol 72, 1245–1254 (2016). https://doi.org/10.1007/s00228-016-2092-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2092-3