Abstract
Purpose
Since the 1970s, the use of metamizole is controversial due to the risk of agranulocytosis. The aim of this study was to analyze individual case safety reports (ICSRs) of metamizole-associated hematological adverse drug reactions (ADRs).
Methods
International and Swiss metamizole-associated ICSR concerning selected hematological ADR were retrieved from VigiBase™, the World Health Organization Global Database of ICSR, and the Swiss Pharmacovigilance Database. We evaluated demographic data, co-medication, drug administration information, dose and duration of metamizole treatment, as well as the latency time of ADR, their course, and severity. The subgroup analysis of Swiss reports allowed us to analyze cases with fatal outcome more in depth and to estimate a rough minimal incidence rate.
Results
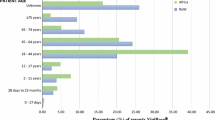
A total of 1417 international and 77 Swiss reports were analyzed. Around 52 % of the international and 33 % of the Swiss metamizole-associated hematological ADR occurred within a latency time of ≤7 days. More women were affected. The annual number of hematological reports and those with fatal outcome increased over the last years parallel to metamizole sales figures. In Switzerland, the minimal incidence rate of agranulocytosis was 0.46–1.63 cases per million person-days of use (2006–2012). Female sex, old age, pancytopenia, and co-medication with methotrexate were striking characteristics of the seven Swiss fatal cases.
Conclusions
Metamizole-associated hematological ADR remain frequently reported. This is underscored by increasing annual reporting rates, which mainly reflect growing metamizole use. Early detection of myelotoxicity and avoidance of other myelotoxic substances such as methotrexate are important measures for preventing fatalities.



Similar content being viewed by others
References
Gierse JK, Koboldt CM, Walker MC, Seibert K, Isakson PC (1999) Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem J 339(Pt 3):607–614
Campos C, de Gregorio R, Garcia-Nieto R, Gago F, Ortiz P, Alemany S (1999) Regulation of cyclooxygenase activity by metamizol. Eur J Pharmacol 378(3):339–347
Hinz B, Cheremina O, Bachmakov J, Renner B, Zolk O, Fromm MF et al (2007) Dipyrone elicits substantial inhibition of peripheral cyclooxygenases in humans: new insights into the pharmacology of an old analgesic. FASEB J 21(10):2343–2351. doi:10.1096/fj.06-8061com
Pierre SC, Schmidt R, Brenneis C, Michaelis M, Geisslinger G, Scholich K (2007) Inhibition of cyclooxygenases by dipyrone. Br J Pharmacol 151(4):494–503. doi:10.1038/sj.bjp.0707239
Rogosch T, Sinning C, Podlewski A, Watzer B, Schlosburg J, Lichtman AH et al (2012) Novel bioactive metabolites of dipyrone (metamizol). Bioorg Med Chem 20(1):101–107. doi:10.1016/j.bmc.2011.11.028
(1986) Risks of agranulocytosis and aplastic anemia. A first report of their relation to drug use with special reference to analgesics. The International Agranulocytosis and Aplastic Anemia Study. JAMA 256(13):1749–1757
Bottiger LE, Westerholm B (1973) Drug-induced blood dyscrasias in Sweden. Br Med J 3(5875):339–343
Hedenmalm K, Spigset O (2002) Agranulocytosis and other blood dyscrasias associated with dipyrone (metamizole). Eur J Clin Pharmacol 58(4):265–274. doi:10.1007/s00228-002-0465-2
Chan TY, Chan AW (1996) Aminopyrine-induced blood dyscrasias—still a problem in many parts of the world. Pharmacoepidemiol Drug Saf 5(4):215–219. doi:10.1002/(SICI)1099-1557(199607)5:4<215::AID-PDS208>3.0.CO;2-5
Zukowski M, Kotfis K (2009) Safety of metamizole and paracetamol for acute pain treatment. Anestezjol Intens Ter 41(3):170–175
Andrade SE, Martinez C, Walker AM (1998) Comparative safety evaluation of non-narcotic analgesics. J Clin Epidemiol 51(12):1357–1365
Solal-Celigny P (1994) Abnormal hematologic values. In: Benichou C (ed) Adverse drug reactions: a practical guide to diagnosis and management. John Wiley & Sons, Chichester, pp 13–30
(1999) Reporting adverse drug reactions. Definitions of terms and criteria for their use. Council for International Organizations of Medical Sciences (CIOMS), Switzerland
Ibanez L, Vidal X, Ballarin E, Laporte JR (2005) Population-based drug-induced agranulocytosis. Arch Intern Med 165(8):869–874. doi:10.1001/archinte.165.8.869
Theophile H, Begaud B, Martin K, Laporte JR, Capella D (2004) Incidence of agranulocytosis in Southwest France. Eur J Epidemiol 19(6):563–565
Maj S, Lis Y (2002) The incidence of metamizole sodium-induced agranulocytosis in Poland. J Int Med Res 30(5):488–495
van der Klauw MM, Goudsmit R, Halie MR, van’t Veer MB, Herings RM, Wilson JH et al (1999) A population-based case-cohort study of drug-associated agranulocytosis. Arch Intern Med 159(4):369–374
Andersohn F, Konzen C, Garbe E (2007) Systematic review: agranulocytosis induced by nonchemotherapy drugs. Ann Intern Med 146(9):657–665
Ibanez L, Vidal X, Ballarin E, Laporte JR (2005) Agranulocytosis associated with dipyrone (metamizol). Eur J Clin Pharmacol 60(11):821–829. doi:10.1007/s00228-004-0836-y
Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA (1993) Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med 329(3):162–167. doi:10.1056/NEJM199307153290303
Andres E, Maloisel F (2008) Idiosyncratic drug-induced agranulocytosis or acute neutropenia. Curr Opin Hematol 15(1):15–21. doi:10.1097/MOH.0b013e3282f15fb9
Swiss product information online. Documed AG, Basel. 2013. www.compendium.ch. Accessed 18 Dec 2013
Moore N, Hall G, Sturkenboom M, Mann R, Lagnaoui R, Begaud B (2003) Biases affecting the proportional reporting ratio (PPR) in spontaneous reports pharmacovigilance databases: the example of sertindole. Pharmacoepidemiol Drug Saf 12(4):271–281. doi:10.1002/pds.848
Backstrom M, Hagg S, Mjorndal T, Dahlqvist R (2002) Utilization pattern of metamizole in northern Sweden and risk estimates of agranulocytosis. Pharmacoepidemiol Drug Saf 11(3):239–245. doi:10.1002/pds.697
Hazell L, Shakir SA (2006) Under-reporting of adverse drug reactions: a systematic review. Drug Saf Int J Med Toxicol Drug Experience 29(5):385–396
Acknowledgments
The authors would like to thank Sara Hult, member of the analysis team of the Uppsala Monitoring Centre, the WHO Collaborating Centre for International Drug Monitoring, for providing the data and for answering our questions. We also would like to thank Guy Levy from the Vigilance Unit of Swissmedic for his collaboration. We thank also Sanofi-Aventis SA, Streuli Pharma AG, and Sintetica SA for providing the sales figures of their metamizole preparations in Switzerland. We thank also the Senglet-Foundation, the foundation for the promotion of young pharmaceutical researchers in Basel, for their financial support.
Author contribution
Performed research: LB, AT, PE, ARB. Analyzed data: LB, AT, MH, SK, ARB. Wrote manuscript: LB, SK, ARB. All authors contributed to the review and final approval of the manuscript.
Conflict of interest
The authors declare that there are no conflicts of interest regarding this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Accompanying statement
The data for this work were obtained from the WHO Collaborating Centre for International Drug Monitoring, Uppsala, Sweden and from the Swiss health authority, Swissmedic, in Berne, Switzerland. Data from spontaneous reporting are inhomogeneous as a result of different reporting policies worldwide and are vulnerable to underreporting and reporting bias. The information contained in this work is therefore not homogeneous, at least with respect to origin and also to likelihood that the pharmaceutical product caused the adverse reaction. The conclusions drawn based on these data do not necessarily represent the opinion of the World Health Organization or of Swissmedic.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 27.0 kb)
Rights and permissions
About this article
Cite this article
Blaser, L.S., Tramonti, A., Egger, P. et al. Hematological safety of metamizole: retrospective analysis of WHO and Swiss spontaneous safety reports. Eur J Clin Pharmacol 71, 209–217 (2015). https://doi.org/10.1007/s00228-014-1781-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1781-z