Abstract
Osteoporosis is a skeletal disease associated with an imbalance between formation and resorption, leading to net loss of bone mass, loss of bone microarchitecture, and development of fractures. Bone resorption is primarily due to an activation of osteoclastogenesis and an increase in receptor activator of nuclear factor kappa-B ligand (RANKL) expression, a cytokine involved in the final pathway of the osteoclast cycle.
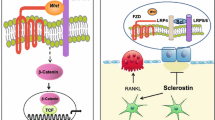
Recent studies of genetic diseases led to the discovery of the wingless-type (Wnt) signaling pathway that plays a major role in bone formation. Further work showed that sclerostin produced by osteocytes and the Dickkopf (DKK1) protein secreted in bone were negative regulators of the Wnt signaling bone formation pathway that act directly by binding to the co-receptors LRP5 and LRP6 of WnT and thereby inhibiting the anabolic Wnt pathway. This understanding of the bone remodeling led to the discovery of new biological drugs that target these pathways and have been evaluated in clinical trials.
The current article discusses the role of these newer “biological” agents in management of osteoporosis. Denosumab, a human monoclonal antibody that specifically binds RANKL, blocks the binding of RANK to its ligand markedly reducing bone resorption, increases bone density, and reduces fractures and is approved for osteoporosis. Parathyroid hormone PTH 1–34 (teriparatide) stimulates bone formation through inhibition of sclerostin, DKK1, and frizzled protein; increases BMD; improves microarchitecture; and decreases fractures and is approved for osteoporosis. The anti-sclerostin antibodies (romosozumab, blosozumab) increase bone mass by neutralizing the negative effects of sclerostin on the Wnt signaling pathway. These biologics are being evaluated now in a clinical trial and early data looks promising. Cathepsin K is a proteolytic enzyme that degrades bone matrix and inhibitors such as odanacatib show increasing bone density and perhaps decreased fractures. The potential power of combining these newer antiresorptives with the newer anabolic agents could theoretically increase bone mass rapidly to normal within 1 year and reduce fractures. These newer treatments are revolutionizing the management of osteoporosis.

Similar content being viewed by others
References
Pacifici R (1996) Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res 11:1043–1051
Riggs BL, Khosla S, Melton LJ III (1998) A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res 13:763–773
Hofbauer LC et al (2000) The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 15:2–12
Shevde NK, Bendixen AC, Dienger KM, Pike JW (2000) Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci U S A 97:7829–7834
Balemans W, Ebeling M, Patel N et al (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543
Loots GG, Kneissel M, Keller H et al (2005) Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res 15:928–935
Li X, Ominsky MS, Niu QT et al (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869
Li X, Ominsky MS, Warmington KS et al (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24:578–588
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Moester MJ, Papapoulos SE, Lowik CW et al (2010) Sclerostin: current knowledge and future perspectives. Calcif Tissue Int 87:99–107
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33(5):747–783. doi:10.1210/er.2011-1060. Epub 2012 Jun 20
Glantschnig H, Hampton RA, Lu P et al (2010) Generation and selection of novel fully human monoclonal antibodies that neutralize Dickkopf-1 (DKK1) inhibitory function in vitro and increase bone mass in vivo. J Biol Chem 285(51):40135–40147. doi:10.1074/jbc.M110.166892
Prolia 60 mg solution for injection in a pre-filled syringe: EU summary of product characteristics. Breda: Amgen Europe B.V., 2010 May 26.
Xgeva (denosumab) injection, for subcutaneous use: US prescribing information. Thousand Oaks (CA): Amgen Inc., 2010.
Miller PD, Bolognese MA, Lewiecki EM et al (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43(2):222–229
McClung MR, Lewiecki EM, Cohen SB et al (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354(8):821–831
Lewiecki EM, Miller PD, McClung MR et al (2007) Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res 22(12):1832–1841
Cummings SR, San Martin J, McClung MR et al (2009) Denosumab for preventionof fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756
H.G. Bone, R. Chapurlat, M.L. Brandi, et al., The effect of thee or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the freedom extension, J. Clin. Endocrinol. Metab. (2013) (Epub ahead of print).
Brown JP, Prince RL, Deal C et al (2009) Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res 24(1):153–161
Kendler DL, Roux C, Benhamou CL et al (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25(1):72–81
Reid IR, Miller PD, Brown JP et al (2010) Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res 25(10):2256–2265
Seeman E, Delmas PD, Hanley DA et al (2010) Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 25(8):1886–1894
Bekker PJ, Holloway DL, Rasmussen AS et al (2004) A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res 19(7):1059–1066
Eastell R, Christiansen C, Grauer A, et al. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res. Epub 2010 Sep 13
Block GA, Bone HG, Fang L et al (2012) A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 27(7):1471–1479. doi:10.1002/jbmr.1613
Neer RM, Arnaud CD, Zanchetta JR et al (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434
Zanchetta JR, Bogado CE, Ferretti JL et al (2003) Effects of teriparatide [recombinant human parathyroid hormone (1–34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res 18:539–543
Greenspan SL, Bone HG, Ettinger MP et al (2007) Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med 146:326
Eastell R, Nickelsen T, Marin F et al (2009) Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European study of Forsteo (EUROFORS). J Bone Miner Res 24:726
Lindsay R, Nieves J, Formica C et al (1997) Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 350:550–555
Cosman F, Nieves J, Woelfert L et al (2001) Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res 16:925–931
Ste-Marie LG, Schwartz SL, Hossain A, Desaiah D, Gaich GA (2006) Effect of teriparatide [rhPTH (1–34)] on BMD when given to postmenopausal women receiving hormone replacement therapy. J Bone Miner Res 21:283–291
Deal C, Omizo M, Schwartz EN et al (2005) Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res 20:1905–1911
Black DM, Greenspan SL, Ensrud KE et al (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215
Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349:1216–1226
Black DM, Bilezikian JP, Ensrud KE et al (2005) One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 353:555–565
J.N. Tsai, A.V. Uihlein, H. Lee, Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial, Lancet 382 (July (9886)) (2013) 50–56, http://dx.doi.org/10.1016/S0140-6736(13)60856-9.
Leder BZ, Tsai JN, Uihlein AV Two years of denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014 Feb 11:jc20134440. [Epub ahead of print]
Kurland ES, Cosman F, McMahon DJ et al (2000) Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069–3076
Orwoll ES, Scheele WH, Paul S et al (2003) The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18:9–17
Forteo (teriparatide [rDNA origin] injection) [package insert]. Product information. Indianapolis, Ind: Eli Lilly and Co; 2002.
Babcook J, Leslie K, Olsen O et al (1996) A novel strategy for generating monoclonal antibodies from single, isolated lymphocytes producing antibodies of defined specificities. Proc Natl Acad Sci U S A 93(15):7843–7848
Veverka V, Henry A, Slocombe P et al (2009) Characterization of the structural features and interactions of sclerostin: molecular insight into a key regulator of Wnt-mediated bone formation. J Biol Chem 284:10890–10900
Ominsky M, Vlasseros F, Jolette J et al (2010) Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25:948–959
Agholme F, Li X, Isaksson H et al (2010) Sclerostin antibody treatment enhances metaphyseal bone healing in rats. J Bone Miner Res 25(11):2412–2418
Li X, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Grisanti M et al (2010) Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass and bone strength in aged male rats. J Bone Miner Res 25(12):2647–2656
Ominsky M, Samadfan R, Jolette J et al (2009) Sclerostin monoclonal antibody stimulates bone formation and improves the strength and density of the fracture callus and lumbar spine in a primate fibular osteotomy model. J Bone Miner Res 24(1):S89–S90
Padhi D, Jang G, Stouch B, Fang L, Posvar E (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26:19–26
McClung M, Grauer A, Boonen S et al (2012) Inhibition of sclerostin with AMG 785 in postmenopausal women with low bone mineral density: phase 2 trial results. J Bone Miner Res 27(1):S8
McClung MR, Grauer A, Boonen S et al (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370(5):412–420. doi:10.1056/NEJMoa1305224. Epub 2014 Jan 1
McColm J, Womack T, Hu L et al (2012) Blosozumab, a humanized monoclonal antibody against sclerostin, demonstrated anabolic effects on bone in postmenopausal women. J Bone Miner Res 27(1):S9
Benson, C., Robins, D., Recker, R., et al. (2013) Effect of blosozumab on bone mineral density: results of a phase 2 study of postmenopausal women with low bone mineral density. Bone Abstracts 1: OC5.3.
Rodan SB, Duong LT (2008) Cathepsin K—a new molecular target for osteoporosis. IBMS BoneKEy 5:16–24
Stoch SA, Zajic S, Stone J et al (2009) Effect of the cathepsin K inhibitor odanacatib on bone resorption biomarkers in healthy postmenopausal women: two double-blind, randomized, placebo-controlled phase I studies. Clin Pharmacol Ther 86:175–182
Bone HG, McClung MR, Roux C (2010) Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J Bone Miner Res 25:937–947
Brixen K, Chapurlat R, Cheung AM et al (2013) Bone density, turnover, and estimated strength in postmenopausal women treated with odanacatib: a randomized trial. J Clin Endocrinol Metab 98:571–580
Eisman JA, Bone HG, Hosking DJ et al (2011) Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res 26:242–251
Tella SH, Gallagher JC (2013) Bazedoxifene + conjugated estrogens in HT for the prevention of osteoporosis and treatment of vasomotor symptoms associated with the menopause. Expert Opin Pharmacother 14(17):2407–2420. doi:10.1517/14656566.2013.844790. Epub 2013 Oct 7
Tella SH, Gallagher JC (2013) Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2013.09.008
Acknowledgments
This work was supported by the National Institute on Aging (RO1-AG28168) and the Office of Dietary Supplements and by a grant from the Department of Defense (DOD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tella, S.H., Gallagher, J.C. Biological agents in management of osteoporosis. Eur J Clin Pharmacol 70, 1291–1301 (2014). https://doi.org/10.1007/s00228-014-1735-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1735-5