Abstract
Floating materials of both natural and anthropogenic origin affect marine ecosystems and human economic activities. Although the tracking of floating materials is important to manage the economic risks, it is difficult to trace them back to the events of origin, such as tsunamis and underwater volcanic eruptions. The gooseneck barnacle Lepas anserifera, a rapid colonizer in pelagic environments, is a potential “natural logger” of floating materials. In this study, we performed temperature-controlled culture experiments and growth line staining in the laboratory to quantify the growth increments of shells (scutum and tergum) consisting the capitulum of L. anserifera separately, and to examine the effects of the temperature on their growth. Following calcein staining, the growth lines of L. anserifera were visualized under a fluorescent microscope, and gross (capitular length and width) and individual (scutum and tergum) shell growth were compared. Shells grew in twice as much in the capitular length direction than in the capitular width direction owing to the larger growth increases in the scutum than in the tergum. Growth increments were unaffected by temperatures in the range from 20°C to 30°C, although the growth appeared to slow down in September and October compared with August. The stable oxygen isotope composition (δ18O) of the shells represented the water temperature as previously known, and the present results showed that 18O enriched in scutum than tergum in most cases. Further understanding for the biomineralization process of barnacles is required for the precise application of environmental proxies in barnacle shells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Floating materials of natural (e.g. pumice and seaweed) and anthropogenic (e.g. plastic) origin provide common habitats for pelagic animals including fish juveniles and fouling animals, such as barnacles and bryozoans, and contribute to pelagic biodiversity and productivity (Sano et al. 2003; Mesaglio et al. 2021). Occasionally, if the number of floats is very large, they may cause unfavorable events ecologically and economically (Hammer et al. 2012; Ohno et al. 2022). Plastics are now widely recognized to act as marine pollutants through various aspects: causing the death of marine animals by entanglement or ingestion; and leaching of toxic materials present in the plastics (United Nations Environment Programme 2005). Owing to their floating nature, with positive buoyancy, and wide distribution range from the coast to offshore, plastics have contributed to the generation of neopelagic communities, an admixture of pelagic and coastal animal communities (Haram et al. 2021). Tsunamis or other unexpected hazards contribute to transport of anthropogenic floating for extraordinary long distances with associating animals, as shown by the 2011 Great East Japan Earthquake and subsequent massive tsunami (Carlton et al. 2017). The pumice raft, which is one of the common natural marine floats occurring on a geological time scale, is known to affect coastal ecosystems through species invasion and human economics by impacting marine traffic (Redick 2023). The submarine volcanic eruption of Fukutoku Oka-no-ba in Ogasawara Arc in August 2021 released huge amounts of pumice; this affected coastal ecosystems, including coral reefs, and economic activities, such as fisheries and ship traffic by causing the formation of pumice rafts, in a large proportion of the Japanese Archipelago (Ohno et al. 2022). To avoid such unfavorable economic problems, tracking and/or forecasting the marine rafts is important.
Satellite tracking and physico-oceanographic simulation are the two main methods used to track and forecast the marine rafts (Nishikawa et al. 2023). The verification of simulated data is usually performed by tracking buoys that are periodically released at a given point (Mitarai et al. 2016). An unexpected drift of an ocean-bottom electromagnetometer covered with sessile animals can provide a serendipitous opportunity to verify the simulation of particles (Tada et al. 2021). As verification opportunities are currently limited, cost-effective and widely available methods are needed for more precise estimation.
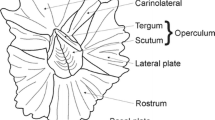
Barnacles have been common sessile animals on marine floats since the Mesozoic era (Gale 2021). The gooseneck barnacles of the genus Lepas are sessile animals that are commonly found on marine floats of both anthropogenic and natural origins, from arctic to tropic oceans worldwide to date (Darwin 1851). The barnacles of the genus Lepas, as well as other barnacles, have calcareous plates (shells) to protect its soft body during epizoic life and are known as a rapid colonizer in pelagic environments (MacIntyre 1966; Mesaglio et al. 2021). Barnacle shells are generally considered to have bilateral symmetry and opercular plates, consisting of the scutum and the tergum, are better indicators of age and growth (Crisp and Bourget 1985).
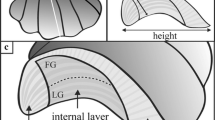
The use of barnacle shells as “natural data loggers” for marine floats has been examined in two main ways. First, the longevity of a floating object can be estimated by measuring the size of attached barnacles. Second, the temperature history experienced by the barnacle on the float can be estimated via measuring the stable oxygen isotope composition (δ18O) of the barnacle shell. The length of the capitulum (capitular length; CL) is a commonly used metric characterizing the size of the barnacles of the genus Lepas. In general, barnacle size gradually increases according to the time elapsed since they settled on the substrate. Therefore, barnacle size may be used to perform rough estimation of the age of the substrate. Moreover, the growth rate of Lepas barnacles varies in response to temperature and food supply and therefore shows seasonal variation (Inatsuchi et al. 2010; Mesaglio et al. 2021; Goehlich et al. 2024). The currently known maximum CL growth rate for Lepas species is 1.45 mm/day, which was observed in a field experiment of L. anserifera (Mesaglio et al. 2021). Despite the fact that the capitular shells of Lepas species comprise five plates, pairs of opercular plates (i.e., the scutum and tergum) and a carina (Fig. 1), the growth patterns of the individual shells remain unknown.
The use of δ18O measurements as a temperature proxy for barnacle shells has been investigated for a wide range of temperatures, from − 2°C to + 26°C (Killingley and Newman 1982; Ullmann et al. 2018; Pearson et al. 2020; Mesaglio et al. 2021; Al-Qattan et al. 2023). δ18O of 11 balanomorph barnacle shells could be defined using a single equation, and these barnacle shells were enriched in 18O compared with molluscs (Killingley and Newman 1982). By contrast, “stalked” barnacles, such as Calantica villosa, Pollicipes polymerus and Neolepas zevinae, showed intermediate values between molluscs and balanomorphs (Killingley and Newman 1982). Furthermore, investigation of the relationship between temperature and δ18O values of the barnacles of the genus Lepas, i.e., “stalked” barnacles from a phylogenetically distinct lineage relative to Calantica and Pollicipes (Chan et al. 2021) revealed slight differences between the species, L. anatifera and L. anserifera (Mesaglio et al. 2021). These investigations were performed to estimate the temperature that the barnacle experienced and to estimate the migration of their substrata using with physical and/or chemical models (Pearson et al. 2020; Al-Qattan et al. 2023). Notably, the barnacle shells comprise multiple plates, and discrete analysis of the shells may improve the accuracy of water temperature estimation and migration tracking.
In this study, we cultured L. anserifera, the dominant species present on pumice rafts in coastal Japan, under temperature-controlled (i.e., 20°C–30°C) conditions. These conditions cover the habitable temperature range of L. anserifera (Inatsuchi et al. 2010). We visualized the specific growth lines of barnacles formed at the start and the end of the culture experiment, as per the method described by Fujikura et al. (2003) and Yusa et al. (2018) to identify the shell increments of the culture period. This identification enabled us to quantify individual and gross shell growth and their specific δ18O under the culture period, and we examined: (1) the relationship between temperature and shell growth in CL; (2) the difference in growth increments between CL and capitular width (CW); (3) the difference in shell growth between individual shells (i.e., the scutum and tergum); and (4) the difference in δ18O values between the scutum and the tergum. Next, we correlated the gross shell growth and individual shell growth of L. anserifera under different temperature conditions and specified the variation of δ18O of the shells of a single individual. Taken together, these procedures permitted to elucidate the characteristics of the shell formation of L. anserifera and discuss its potential application as a natural data logger.
Materials and methods
Culture experiment: Eighty-one individuals of L. anserifera drifted ashore on Minami-hama beach, located beside the Seto Marine Biological Laboratory, Kyoto University, on August 5, 2021, were used for the culture experiment. The specimens were cultured in a 60 L aquarium tank with flow-through seawater (300 mL/min) pumped up from the shore to the tank to remove suspensions and provided to laboratory. About 2 g of brine shrimp cysts were directly added to the aquarium twice a week as it hatched within a day and 2 g of crushed dried krill fed between the brine shrimp feedings. Each feed was removed from the aquarium before the next feeding. On August 28, 2021, 81 individuals were immersed for 24 h in seawater containing calcein (0.7 g/L) that allows us to observe mm-scale growth increments under fluorescence microscopy and SrCl2 (2.88 g/L) for μm-scale growth increments under SEM, to stain their growth lines, according to the previous studies (Fujikura et al. 2003; Yusa et al. 2018) and our unpublished experiments. After staining, the specimens were separated into three groups and transferred into separate aquarium tanks at different temperature conditions: without any cooling/heating (Room); cooling down to 20 °C (Cool); and heating up to 30 °C (Heat), with flow-through natural seawater and feeding as in the preexperiment, although the brine shrimp fed in the 20 °C aquarium was hatched in another aquarium at the room temperature before feeding. To precisely identify the growth during the experimental period, we performed a second staining, 20 days after the first one (September 13–14, 2021) using the same procedure as for the first staining, and cultured in the three flow-through aquariums to October 25, 2021. Temperature and salinity of each aquarium tank were measured every 30 min using a Horiba D-200 or JFE Advantech COMPACT-CTD Lite. The salinity was corrected by temperature because it was calculated from the conductivity. Approximately half of the individuals died after the first staining, and this suggests that something in the staining process may have affected the mortality rates. However, almost all the individuals survived after the second staining on September 14 (Fig. 2c), and we only used the surviving specimens for the following analyses.
Size and growth increment measurement: Sizes of the specimens, determined by capitular length (CL) and capitular width (CW; Fig. 1), were measured manually using calipers for all living specimens before the first staining (August 28), the second staining (September 14), and at the end of the culture experiments (October 25). After the culture period, on October 25, 2021, the surviving specimens were immediately dissected and the shells were detached.
The shells were carefully washed with Milli-Q water and air-dried under dark conditions at JAMSTEC before observing the calcein-marked growth lines using a fluorescent binocular stereo microscope (Olympus SZX7). The length between two fluorescent growth lines along the maximum growth axis was measured to quantify the growth rate of each part of the shell (scutum, tergum, and carina) over 20 days. Photographic images were obtained using a single lens reflex camera (Panasonic DMC-GH3) mounted on a fluorescence binocular microscope. To visualize the fluorescent growth lines more clearly than in a previous study (Yusa et al. 2018), the color of the fluorescent image was inverted by Photoshop 2023 (Adobe Inc., 2023) and overlaid on the epi-illuminated image of the shell without fluorescence. The shell increments between two fluorescent growth lines and between the second fluorescent growth line and edge of the shells were measured from the images using ImageJ software (Schneider et al. 2012). All measurements were performed three times, and the mean value was used as the growth increment. Unfortunately, other markings by SrCl2 could not be observed under SEM (Hitachi TM-3000); however, the growth increments were large enough to be measured under the fluorescence microscope.
Statistical analyses: The effect of temperature on shell increments was analyzed from three perspectives: size (CL and CW) at a certain date, increments of CL and CW, and individual shell increments of the scutum and tergum. All analyses were fit to either a generalized linear model (GLM) or a generalized linear mixed model (GLMM; Stroup 2012) as implemented by the GLIMMIX procedure of SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). First, CL and CW were analyzed with a GLMM using a gamma distribution and ln-link function, with the temperature condition (Cool, Room, or Heat), observation date (first staining in August, second staining in September, or the end of the experiments in October), and their interactions as categorical factors, and individual identification number as a random factor. Second, the growth increments of CL and CW were analyzed with a GLM using a gamma distribution and ln-link function, with temperature as a categorical factor. Finally, the growth increments of the plates were analyzed with a GLMM using a gamma distribution and ln-link function, with temperature, plate (scutum or tergum), and their interactions as categorical factors, growth increments of CL as a covariate, and individual identification number as a random factor. In all cases, the method of Kenward and Roger (1997) was used to adjust the (possibly fractional) denominator degrees of freedom for F tests to account for the estimated correlated responses. To facilitate interpretation, we present the results for a particular factor or a covariate adjusted for the effects of other components of the statistical models. For categorical factors, we present least-squares means and their standard errors (Milliken and Johnson 2009). All differences among variables were examined by the Tukey–Kramer multiple comparison test.
Stable oxygen isotope composition: The temperature of the culture conditions was relatively stable between the first and second staining but varied after the second staining owing to the temperature depletion and typhoon effects in natural seawater (Table 1 and Fig. 2). Therefore, the shells formed between the first and second staining were milled and used to measure the stable oxygen isotope composition. Scutum and tergum of two specimens from each of the three culture conditions were measured from more than three separate samples. The carina was not used in this analysis as the carina of all specimens was too small to obtain multiple samples for the analysis. The powdered shell samples were analyzed using an isotope ratio mass spectrometer (IsoPrime, Micromass) at JAMSTEC. The stable oxygen isotope composition (δ18O) of shells was reported relative to Pee Dee Belemnite. The analytical precision (1σ) was better than ± 0.1 ‰ in all cases.
Results
Size and growth increment measurement: Measurements of the capitular parameters CL and CW showed that sizes increased during the culture experiment, with growth increments of up to 0.33 mm/day (Supplementary Table S1). CW was generally larger than CL, and the growth increments of CW were approximately twice those of CL in most individuals (Fig. 3a). The effects of temperature and observation date on CL and CW were examined by GLMM as the first statistical analysis, and only observation date was found to be significant for both CL and CW (CL: F(2, 119) = 6.29, P < 0.01, CW: F(2, 119) = 6.03, P < 0.01; Table 2). Both CL and CW were significantly larger after the second staining (September 14 and October 25) than after the first staining (August 28) at all temperatures (Fig. 4). The effects of temperature on growth increments were significant for CL, as growth increments of CL were smaller under cool conditions than under room/heat conditions (F(2, 26) = 6.12, P < 0.01), but there was no significant effect for CW (F(2, 24) = 1.13, P = 0.34; Fig. 5, Table 3).
Difference in size in CL (a) and CW (b) among the dates of measurements. Red: Heat conditions, blue: Cool conditions, and gray: Room conditions. Error bars show the standard errors. Letters below the symbols indicate statistical significant differences supported by the Tukey–Kramer multiple comparison test. **P < 0.01
The growth lines on the shells (scutum and tergum) of L. anserifera were successfully stained and observed under a fluorescent microscope (Supplementary Figs. S1 and S2). The growth increments between the two stained growth lines and between the second stained line and the edge of the shells were measured for the scutum and tergum (Supplementary Table S2). The growth rates of the scutum were approximately twice those of tergum (Fig. 6). GLMM analysis showed that the growth increments were significantly different between the scutum and the tergum and among the three temperatures, although the interaction between temperature and type of shell was not significant (Table 4, Type of shell: F(1, 3.987) = 28.17, P < 0.01, Temperature: F(2, 8.541) = 32.94, P < 0.001, Temperature × Type of Shell: F(2, 3.948) = 1.92, P = 0.26; Fig. 7). The growth increments in scutum and tergum under room temperature conditions were significantly smaller than those under other temperature conditions (Fig. 7), whereas gross shell increments (CL and CW) were not (Fig. 5).
Difference in growth increments between the least-square means of the scutum and tergum (a) and among the temperatures (b). Error bars indicate the standard errors. Different letters beside the symbols indicate statistically significant differences supported by the Tukey–Kramer multiple comparison test. **P < 0.01
Stable oxygen isotope composition: The δ18O measurements varied within the same individual; the values were relatively lower in the tergum and higher in the scutum, except for a single individual labeled as Heat 24 (Supplementary Figure S3, Table S3). The temperature estimates, based on the equation provided by Mesaglio et al. (2021), were almost consistent with the temperature conditions and the estimates from the equation are presumed to be useful enough; however, the present results provide separate equations for scutum and tergum to estimate the temperature based on the δ18O (Fig. 8);
where temperature is the water temperature in which the L. anserifera sample is immersed, δ18Os and δ18Ot are the δ18O of the scutum and tergum, respectively, and ε is the error. In the maximum case, the estimated temperature differed by approximately 1 °C between scutum and tergum (Fig. S3).
Discussion
Temperature effects on shell growth
The present culture experiments revealed the characteristic shell growth in L. anserifera. Temperature was initially hypothesized to be the main factor controlling the shell growth; however, the shell size increased gradually as time passed in the experiment and significant growth was only observed between the first staining (August 28) and the second staining (September 14), irrespective of the temperature conditions (Fig. 4). Although more individuals were required to obtain more accurate results through statistical analysis, we have identified some trends in the shell growth from these results. Food availability is one of the plausible factors affecting the growth of L. anserifera (Inatsuchi et al. 2010), as mentioned by Goehlich et al. (2024) for Lepas spp. Although the feeding (hatched brine shrimp and crushed krill) was kept constant throughout the experimental period, seawater conditions were kept changing. The Seto Marine Biological Laboratory, where this culture experiment was performed, is on the western side of the Kii peninsula, facing Kuroshio. The seasonal variation in chlorophyll a concentration—one of the proxies for primary production in the ocean—along Kuroshio is high in winter (October to June) and low in summer (July to September) (Miyashita 2005). Our results indicate that other factors rather than temperature should be considered as a factor controlling the shell growth of L. anserifera. Finally, no seasonal effect in shell growth was found in the other barnacles (e.g. Pollicipes, as summarized in Cruz et al. 2022), and shell growth does not always reflect their habitat conditions.
Growth rate difference between CL and CW
The maximum growth rate of CL observed in this study was 0.33 mm/day. The maximum growth rate of L. anserifera was reported as 1.45 mm/day in a field experiment (Mesaglio et al. 2021), more than four times larger than the maximum growth rate observed in the present study. The growth rate of L. anserifera varies according to its growth stage: rapid growth (0.83 mm/day on average) in the early stage (up to 15 days; approximately 12 mm in CL) and slow growth (0.39 mm/day on average) in the later stage (15–42 days; approximately 15 mm in CL) (Inatsuchi et al. 2010). The specimens used for the present study were relatively large (CL of more than 10 mm) and thus the growth rate was comparatively smaller, although a growth rate of approximately 0.33 mm/day is still quite rapid among barnacles (Crisp and Bourget 1985).
Interestingly, the stagnant growth of L. anserifera seems to be only observed after summer except for the data obtained by Mesaglio et al. (2021). The growth experiments in both Inatsuchi et al. (2010) and the present study were performed at the Seto Marine Biological Laboratory, and the primary production of the ocean appears to be low in summer; August and September (Miyashita 2005). Hoek (1907) reported the growth of L. anserifera in near-equatorial Indonesian water. One possibility is that the seasons are reversed in the southern and northern hemispheres; however, a higher growth rate was reported (1.05 mm/day) in austral summer (January and February, Mesaglio et al. 2021). Reproduction is another possibility for the stagnant growth of the shell. Because of the difficulty in generalizing the amount of shell growth of L. anserifera, estimating the age of L. anserifera and the longevity of its floating substrata from the size of the gross shell growth (CL and CW) is difficult.
Differences in growth between the scutum and the tergum
The growth lines that were visible under the fluorescence microscope enabled us to identify growth increments for the shells of L. anserifera (Fig. 6). The growth increments were different between the scutum and the tergum, which contributed to the two-fold greater growth in the CL direction than in the CW direction. This study provides the first insight to connect the individual shell growth observed in the scutum and tergum with the growth in CL and CW. The effects of temperature on shell increments in scutum and tergum were not observed (Fig. 7), whereas the shell increments in scutum and tergum were correlated (Fig. 6) and amount of the growth increments were not the same in all temperatures and between culture periods (Supplementary Figs. S1 and S2). The shell growths of scutum and tergum of L. anserifera were not quantified and generalized in the present study, and using the shell growth to estimate the age of L. anserifera and the longevity of its floating substrata is difficult, as observed using gross shell growth. Although it is faint, several growth lines can be observed on the surface of the shells (Supplementary Figs. S1 and S2). Quantifying the interval between the formation of the growth lines enables us to estimate the age of L. anserifera and the longevity of its floating substrata.
Stable oxygen isotope composition and shell growth
The stable oxygen isotope composition of the shells of L. anserifera appear to represent the temperature of their habitat, as previously reported (Mesaglio et al. 2021). The previous study used the tip of the scutum, regardless of the growth lines, which may not represent the same temporal dimension on the shell. The present study identified the specific shell regions grown under the controlled conditions by the two stained growth lines visible under a fluorescent microscope and obtained the stable oxygen isotope ratio of the barnacle shells according to the temperature variations. The results of the stable oxygen isotope composition in the present study and Mesaglio et al. (2021) were almost the same, although the temperature fluctuations in the field experiments by Mesaglio et al. (2021) were much larger than those in the present study performed under laboratory conditions. Sampling the specific region along the growth line improved the accuracy of the stable isotopic data of shells.
The δ18O values of the scutum and tergum of a single individual were not consistent; 18O was generally enriched in the scutum compared with the tergum, except for a single individual, and separate equations to estimate water temperature were suggested (Fig. 8). As the growth increments were significantly larger in the scutum than in the tergum (Fig. 7a), the isotopic composition may reflect the growth difference between the two shells. The δ18O measured for hard parts of marine animals can, in some cases, be variable, such as among the different shell layers in a single individual of brachiopods (Rollion-Bard et al. 2019). Alternatively, the δ18O values of the scutum, basis and inner sheath of the sessile barnacle Menesiniella aquila were measured along with the growth directions of each shell and no specific trend was detected (Killingley and Newman 1982). Although the shell secretion process used by barnacles remains unclear, Killingley and Newman (1982) predicted the existence of important differences in the process used by molluscs and barnacles based on observed differences in δ18O values, as well as the existence of phylogenetic constraints among barnacle orders. Recent genomic analyses have revealed both similarities and dissimilarities in gene evolution associated with biomineralization between molluscs and barnacles (Yuan et al. 2022). Further examination of the biomineralization process in barnacles is required to understand why δ18O differs within a single individual in the present study and to use the barnacle shells as a proxy for water temperature in the future.
To apply barnacle shells as a natural data logger, understanding the process of growth line formation on the shell as well as identification of the shell is necessary. The growth lines were faintly observed on the shells of L. anserifera in this study, and most of them were hardly counted. Growth lines on shells of balanomorph barnacles are known to be reduced or concentrated by environmental stress (Bourget and Crisp 1975). The specimens used in this study were large enough to reproduce according to Inatsuchi et al. (2010), and reproduction might cause the growth stagnant. In most cases, the shells of L. anserifera are very smooth and some growth lines were barely observed. Furthermore, the phylogenetic constraints of shell formation process suggested by Killingley and Newman (1982) may cause the differences in shell growth line formation. Visualizing the calcification process under microscopy (Toyofuku et al. 2017), as well as genetic analyses, will help us to describe the formation process of growth lines on barnacle shells, and the differences in the δ18O between the scutum and the tergum.
Conclusion
This study showed heterogeneity of shell formation, including the disagreement of δ18O between the shells of a single individual of L. anserifera. The growth rate of barnacles may not be affected by temperature, although it is highly variable among individuals both within the same population as well as among the populations (Inatsuchi et al. 2010, Mesaglio et al. 2021, and present study). The δ18O values of barnacle shells actually represented the temperature of their habitat. In addition, the relationship between δ18O and temperature was slightly different in the scutum and tergum, although no clear differences were observed among the shells of balanomorph barnacles (Killingley and Newman 1982). The opercular plates, consisting of the scutum and the tergum, are good indicators of barnacle growth, although the growth rates were not the same in the scutum and tergum of L. anserifera, unlike that previously expected from balanomorphs (Crisp and Bourget 1985). The heterogeneous effects with respect to shell growth in L. anserifera will help us to understand the growth patterns of barnacle shells to form the specific morphology and to apply barnacle as the natural data logger on marine floats. Analyzing the shell plate separately and separate sampling along growth direction are highly important for tracking the trajectories of marine floats or substrates of L. anserifera, as illustrated in Al-Qattan et al (2023) on L. anatifera. However, as the size of the L. anserifera may not simply correlate with its chronological age, the use of L. anserifera as a natural data logger required the visualization of growth lines and the identification of growth line intervals in Lepas species; overall this process is similar to how balanomorph barnacles are studied (Crisp and Bourget 1985). Finally, further research designed to elucidate the mechanism involved in the shell formation process and resulting phylogenetic constraints of barnacles is required.
Data availability
The data are available as the figures and the the tables in Supplementary Information.
References
Al-Qattan N, Herbert GS, Spero HJ, McCarthy S, McGeady R, Tao R, Power AM (2023) A stable isotope sclerochronology-Based forensic method for reconstructing debris drift paths with application to the MH370 Crash. AGU Adv. https://doi.org/10.1029/2023AV000915
Bourget E, Crisp DJ (1975) An analysis of the growth bands and ridges of barnacle shell plates. J Mar Biol Ass UK 55:439–461. https://doi.org/10.1017/S0025315400016052
Carlton JT, Chapman JW, Geller JB, Miller JA, Carlton DA, McCuller MI, Treneman NC, Steves BP, Ruiz GM (2017) Tsunami-driven rafting: transoceanic species dispersal and implications for marine biogeography. Science 357:1402–1406. https://doi.org/10.1126/science.aao1498
Chan BKK, Dreyer N, Gale AS, Glenner H, Ewers-Saucedo C, Pérez-Losada M, Kolbasov GA, Crandall KA, Høeg JT (2021) The evolutionary diversity of barnacles, with an updated classification of fossil and living forms. Zool J Linn Soc 193:789–846. https://doi.org/10.1093/zoolinnean/zlaa160
Crisp DJ, Bourget E (1985) Growth in barnacles. Adv Mar Biol 22:199–244. https://doi.org/10.1016/S0065-2881(08)60052-8
Cruz T, Jacinto D, Fernandes JN, Seabra MI, Van Syoc RJ, Power AM, Macho G, Sousa A, Castro JJ, Hawkins SJ (2022) Pedunculate cirripedes of the genus Pollicipes: 25 years after Margaret Barnes’ review. Oceanogr Mar Biol Ann Rev 60:19–168. https://doi.org/10.1201/9781003288602-3
Darwin C (1851) A monograph of the sub-class Cirripedia, with figures of all the species. The Lepadidæ; or pedunculated cirripedes, vol 1. The Ray Society, London
Fujikura K, Okoshi K, Naganuma T (2003) Strontium as a marker for estimation of microscopic growth rates in a bivalve. Mar Ecol Prog Ser 257:295–301. https://doi.org/10.3354/meps257295
Gale AS (2021) The thoracican cirripede genus Concinnalepas Gale, 2014 (Crustacea) from the Middle and Upper Jurassic of southern England and northern France. Proc Geologists’ Ass 132:332–345. https://doi.org/10.1016/j.pgeola.2021.01.007
Goehlich H, Luna-Jorquera G, Picard A-PD, Pantoja J, Tala F, Thiel M (2024) Seasonal growth rates of gooseneck barnacles (Lepas spp.): Proxies for floating time of rafts in marine ecosystems. Mar Biol 171:36. https://doi.org/10.1007/s00227-023-04336-8
Hammer J, Kraak MHS, Parsons JR (2012) Plastics in the marine environment: The dark side of a modern gift. Rev Environ Contam Toxicol 220:1–44. https://doi.org/10.1007/978-1-4614-3414-6_1
Haram LE, Carlton JT, Centurioni L, Crowley M, Hafner J, Maximenko N, Murray CC, Shcherbina AY, Hormann V, Wright C, Ruiz GM (2021) Emergence of a neopelagic community through the establishment of coastal species on the high seas. Nat Commun 12:6885. https://doi.org/10.1038/s41467-021-27188-6
Hoek PPC (1907) The Cirripedia of the Siboga-expedition. EJ Brill, Leiden
Inatsuchi A, Yamato S, Yusa Y (2010) Effects of temperature and food availability on growth and reproduction in the neustonic pedunculate barnacle Lepas anserifera. Mar Biol 157:899–905. https://doi.org/10.1007/s00227-009-1373-0
Kenward MG, Roger JH (1997) Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997. https://doi.org/10.2307/2533558
Killingley JS, Newman WA (1982) 18O fractionation in barnacle calcite: a barnacle paleotemperature equation. J Mar Res 40:893–902
MacIntyre RJ (1966) Rapid growth in stalked barnacles. Nature 212:637–638. https://doi.org/10.1038/212637a0
Mesaglio TP, Schilling HT, Adler L, Ahyong ST, Maslen B, Suthers IM (2021) The ecology of Lepas-based biofouling communities on moored and drifting objects, with applications for marine forensic science. Mar Biol 168:21. https://doi.org/10.1007/s00227-021-03822-1
Milliken GA, Johnson DE (2009) Analysis of messy data volume 1. Designed experiments, 2nd edn. Chapman and Hall, New York. https://doi.org/10.1201/EBK1584883340
Mitarai S, Watanabe H, Nakajima Y, Shchepetkin AF, McWilliams JC (2016) Quantifying dispersal from hydrothermal vent fields in the western Pacific Ocean. Proc Natl Acad Sci U S A 113:2976–2981. https://doi.org/10.1073/pnas.1518395113
Miyashita M (2005) Bi-weekly to seasonal variability of satellite-derived chlorophyll a distribution: controlling factors in the ocean south of honshu Island. J Remote Sens Soc Jpn 25:169–178. https://doi.org/10.11440/rssj1981.25.169
Nishikawa H, Kuwatani T, Tada N, Kayama Watanabe H (2023) Simulated distributions of pumice rafts in Japan following eruptions at volcanic islands and submarine volcanoes. Prog Earth Planet Sci 10:21. https://doi.org/10.1186/s40645-023-00552-4
Ohno Y, Iguchi A, Ijima M, Yasumoto K, Suzuki A (2022) Coastal ecological impacts from pumice rafts. Sci Rep 12:11187. https://doi.org/10.1038/s41598-022-14614-y
Pearson RM, van de Merwe JP, Connolly RM (2020) Global oxygen isoscapes for barnacle shells: Application for tracing movement in oceans. Sci Total Env 705:135782
Redick NR (2023) A review of pumice raft formation environments, saturation, and dispersal mechanisms. McGill Sci Undergrad Res J 18:B19–B25. https://doi.org/10.26443/msurj.v18i1.187
Rollion-Bard C, Milner Garcia S, Burckel P, Angiolini L, Jurikova H, Tomašových A, Henkel D (2019) Assessing the biomineralization processes in the shell layers of modern brachiopods from oxygen isotopic composition and elemental ratios: Implications for their use as paleoenvironmental proxies. Chem Geol 524:49–66. https://doi.org/10.1016/j.chemgeo.2019.05.031
Sano M, Omori M, Taniguchi K (2003) Predator-prey systems of drifting seaweed communities off the Tohoku coast, northern Japan, as determined by feeding habit analysis of phytal animals. Fish Sci 69:260–268
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to imageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Stroup WW (2012) Generalized Linear Mixed Models: Modern concepts, methods and applications. CRC Texts in Statistical Science. Chapman and Hall, New York
Tada N, Nishikawa H, Ichihara H, Watanabe HK, Kuwatani T (2021) Drift of an ocean bottom electromagnetometer from the Bonin to Ryukyu Islands: Estimation of the path and travel time by numerical tracking experiments. Earth Planets Space 73:224. https://doi.org/10.1186/s40623-021-01552-8
Toyofuku T, Matsuo MY, de Nooijer LJ, Nagai Y, Kawada S, Fujita K, Reichart GJ, Nomaki H, Tsuchiya M, Sakaguchi H, Kitazato H (2017) Proton pumping accompanies calcification in foraminifera. Nat Commun 8:14145. https://doi.org/10.1038/ncomms14145
Ullmann CV, Gale AS, Huggett J, Wray D, Frei R, Korte C, Broom-Fendley S, Littler K, Hesselbo SP (2018) The geochemistry of modern calcareous barnacle shells and applications for palaeoenvironmental studies. Geochim Cosmochim Acta 243:149–168. https://doi.org/10.1016/j.gca.2018.09.010
United Nations Environment Programme (2005) Marine litter: an analytical overview. https://www.unep.org/resources/report/marine-litter-analytical-overview. Accessed 6 June 2024
Yuan J, Zhang X, Li S, Liu C, Yu Y, Zhang X, Xiang J, Li F (2022) Convergent evolution of barnacles and molluscs sheds lights in origin and diversification of calcareous shell and sessile lifestyle. Proc R Soc B 289:20221535. https://doi.org/10.1098/rspb.2022.1535
Yusa Y, Yasuda N, Yamamoto T, Watanabe HK, Higashiji T, Kaneko A, Nishida K, Høeg JT (2018) Direct growth measurements of two deep-sea scalpellid barnacles, Scalpellum stearnsii and Graviscalpellum pedunculatum. Zool Stud 57:e29. https://doi.org/10.6620/ZS.2018.57-29
Acknowledgements
We appreciate Dr. Kennichi Sasaki of the Mutsu Institute of JAMSTEC for kindly providing us with the CTD to measure the environments in the experimental aquaria. We are also grateful to Asami Kajimoto at Nara Women’s University and the staff at the Seto Marine Biological Laboratory of Kyoto University for helping us to carry out the culture experiments.
Funding
This study was supported by CREST (Grant Number JPMJCR1761) from the Japan Science and Technology Agency (JST), and JSPS KAKENHI Grant Number JP24K01139.
Author information
Authors and Affiliations
Contributions
HKW, NT, HN, TK and YY drafted the research plan including the culture experiments and stable isotopic usage. GK and LY carried out the culture experiments and bulk measurements of the shells, in addition to the environmental measurements. YN, TH and HKW carried out the individual shell observation and measurement using the fluorescent microscope and scanning electron microscope, and SS conducted the stable oxygen isotope analyses. YY and HU conducted the statistical analyses. All the authors discussed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant finencial or non-finencial interests to disclose.
Ethical approval
This study did not require ethics approval.
Additional information
Responsible Editor: A. Checa .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Watanabe, H.K., Nagai, Y., Sakai, S. et al. Heterogeneous shell growth of the neustonic goose barnacle Lepas anserifera. Mar Biol 171, 161 (2024). https://doi.org/10.1007/s00227-024-04481-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04481-8