Abstract
In recent years, population outbreaks of the annelid Hermodice carunculata (Polychaeta, Amphinomidae) are recurrently detected along the coastal zone of the Salento peninsula (Southern Italy), with impacts on marine benthic ecosystems. Annelida are renowned for their remarkable regeneration potential, enabling them to reform lost body parts. A handful of studies have reported posterior regeneration of H. carunculata, but anterior regeneration has not been fully explored. In this study, we investigated the capacity of H. carunculata collected in shallow coastal areas (Ionian Sea, 40°08’26.9” N 17°58’44.1” E) to regenerate anterior body parts under different temperature conditions (22 and 14 °C) and considering two different body sizes (∼ 4 g and 25 g). In addition, histological analysis and lipid analyses were carried out to detect changes in the reproductive cycle and lipid storage during ongoing regeneration. The results suggest that small and large-sized specimens of H. carunculata can regenerate efficiently anterior body parts in 12–20 weeks post amputation when kept at 22 °C. Small-sized worms kept at 14 °C regenerated slower but died in 24 weeks post amputation before regenerating a mouth, while large-sized worms kept at 14 °C were affected by a 100% mortality during blastema formation. In addition, lipid extraction analyses show that H. carunculata can regenerate during extended periods of starvation by de novo synthesizing lipid reserves and regeneration in H. carunculata does not negatively impact the reproductive cycle, as gametogenesis occurs also during the regenerative processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bearded fireworm Hermodice carunculata (Pallas, 1766) is a large polychaete found in warm and temperate areas of the Atlantic Ocean, Caribbean Sea and the Red Sea. It is known to inhabit the pre-coralligenous and coralligenous formations in the southern Mediterranean Sea (Fishelson 1971; Righi et al. 2020). Mounting evidence indicates an increase in the abundance of this species within the Mediterranean basin, with a northward expansion most likely driven by rising average water temperatures. Indeed, this species requires a relatively high temperature to complete its life cycle (Righi et al. 2020; Toso et al. 2020). Recent studies unveiled the widespread occurrence of H. carunculata, with tendencies to form dense aggregations in specific areas of the Mediterranean Sea, which classifies it as invasive (Celona and Comparetto 2010; Righi et al. 2020; Schulze et al. 2017; Toso et al. 2022; Tiralongo et al. 2023). Some studies suggested that H. carunculata fireworms may pose a potential threat to coastal marine ecosystems since they are large and mobile omnivores. The increases in fireworm abundance could lead to unusual predatory pressure on various benthic species, such as Cnidarians mollusk and echinoderms (Lewis and Crooks 1996; Barroso et al. 2016; Simonini et al. 2018; Toso et al. 2022). In the Mediterranean Sea, H. carunculata is notably infamous for its ability to consume fish caught in fishing nets. This local phenomenon has resulted in a significant reduction in income for small artisanal fisheries (Celona and Comparetto 2010; Tiralongo et al. 2023). Moreover, in addition to the need for more extensive research to fully evaluate its impact on hard-bottom marine communities, the bearded fireworm is recognized as a hazard to swimmers. Direct contact with human skin can result in painful burns due to the penetrating bristles that inject toxins (Nakamura et al. 2008; Righi et al. 2022). This may potentially affect the tourism, impacting local economies in areas where it is very abundant. Several studies have addressed the biology and the ecology of this species, such as its trophic requirements (Simonini et al. 2017, 2018), ecology and biogeographical distribution (Righi et al. 2019, 2020; Krželj et al. 2020; Toso et al. 2022), ecological plasticity (Grimes et al. 2020; Lucey et al. 2020), life cycle (Toso et al. 2020), and the ability to regenerate posterior body parts (Ahrens et al. 2013, 2014). The ability of replacing body structures and asexual reproduction by transverse fission are highly related, often associated (but not equivalent) developmental processes, with distinct functions: repair or numerical increase the segments of the individuals (Zattara and Bely 2016). However, asexual reproduction in H. carunculata has not been investigated so far, nor has its anterior regenerative potential, as previous studies have solely focused on posterior regeneration and sexual reproduction (Ahrens et al. 2014; Toso et al. 2020).
Regeneration of segments in Annelida is common, although it highly varies across families (Bely 2006, 2010) and it usually occurs after accidental injury or predation (Bely and Nyberg 2010). Posterior segment regeneration appears to be nearly universal within the phylum, while the ability to regenerate anteriorly is less common (Bely 2006, 2010; Brockes and Kumar 2008; Zoran 2010; Licciano et al. 2012, 2015). In some taxa, a high regenerative potential is key for the asexual reproduction by architomy, i.e. fragmentation in two or more incomplete body parts (David and Williams 2012), each of them eventually restored to individual morphs by means of regenerative processes. Other groups (e.g., Eunicida and Syllidae) have sexual reproductive modes (schizogamy or stolonization) requiring posterior regeneration (Franke 1999; Ribeiro et al. 2018; Langeneck et al. 2020). All the above-mentioned forms of regeneration exhibit the same morphogenic patterns of genetic regulation (Bely and Wray 2001; Gibson and Peterson 2003; Bely 2006). In the regenerative processes of annelids linked to asexual reproduction, several endogenous factors act as regulators through both paratomy (i.e., new individuals complete their development before splitting by fission) and/or architomy (Bely 2006; Kostyuchenko and Kozin 2020; Fusco and Minelli 2023). The size class can also regulate the timing of the regeneration process, as in the case of Myxicola infundibulum (Montagu, 1808), where small-size worms regenerate faster than large worms (Prentiss et al. 2017). The interest in regenerative ability of investigated species other than for ecological purposes, could be indicative also for studying the evolution of regeneration within the Annelida, where regeneration is likely to represent an ancestral feature, probably lost several times (Bely and Sikes 2010).
The Amphinomidae family, located at the base of the annelid phylogeny (Weigert and Bleidorn 2016) can represent the perfect model for studying the evolution of regenerative ability along the annelid phylogeny. Within amphinomids, bidirectional (i.e., anterior and posterior) regeneration was observed in two species, which can reproduce asexually, namely Eurythoe complanata (Pallas, 1766) and Linopherus canariensis Langerhans, 1881 (Kudenov 1974; Muller et al. 2003; Cosentino and Giacobbe 2011; Yáñez-Rivera et al. 2014; Weidhase et al. 2016).
Regarding anterior regeneration, the ex-novo production of the anterior part of the body, including the head and the mouth, is required in starving fragments that rely on their energetic reserves. Lipids are important biomarkers in the physiology of marine animals, as they are the major source of metabolic energy and essential for the formation of cell and tissue membranes (Giese 1966; Morris and Sargent 1973; Sargent 1995; Rossi et al. 2017). For example, during anterior regeneration, E. complanata invests a higher quantity of energy to repair the lost body parts, enabling them to withstand at least 50 days of starvation, accompanied by the depletion of lipid concentrations in the tissues (Yáñez-Rivera et al. 2014). The amount of stored lipids depends on the stage of the life cycle, as well as on individual behaviour since many invertebrates often undergo starvation periods (Giese 1966). Moreover, individual size may determine differences in lipid content, as observed in Arenicola marina (Linnaeus, 1758), with small, sexually immature worms having higher lipid content compared to mature, larger worms, as juveniles utilize lipids as reserves for migration (De Vooys 1975).
This suggests that the quantity of stored lipids and sexual reproduction might influence regeneration in polychaetes. In this work, we carried out experimental amputation of the anterior end (prostomium plus the first 6 or 10 segments segments) to investigate (a) the head regeneration process in H. carunculata and its relationship with temperature and body size, and (b) how the regenerative process may affect lipid content and gametogenesis in reared individuals.
Materials and methods
Study site and sample collection
Specimens of Hermodice carunculata were collected on hard rocky bottoms at shallow depths (3–10 m) in the coastal area of Santa Caterina (Ionian Sea, 40°08’26.9” N 17°58’44.1” E). The selected area of sampling is characterized by high density for H. carunculata, up to 1,2 ind/ m2 in the warm season (Toso et al. 2022). Specimens were collected in November 2020, when worms are commonly in a spent phase of the reproductive cycle (Toso et al. 2020). Ten specimens ranging from 3.5 to 35 g in weight were fixed in 4% formalin solution immediately after collection, for the analysis of the status of sexual maturity by histological sections. Sixty specimens of the same weight range were transported to the laboratory and used for the head regeneration experiment.
Experimental setup
Two thermostatic rooms were set at 22 °C and 14 °C, where worms were maintained in 30 l aerated aquaria, at salinity 37‰, and with a 12-h light-dark cycle.
Sixty worms were divided into 6 aquaria (three maintained at 22 °C and three at 14 °C, respectively) according to their size (small: 36 worms ∼ 4 g; large: 24 worms ∼ 25 g) so that each aquarium contained 10 worms (6 small plus 4 large sized worms). Small and large worms reared at 22 °C (warm treatment) are referred to as SW and LW respectively, whereas small and large specimens reared at 14 °C (cold treatment) are referred to as SC and LC.
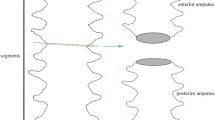
These worms were anesthetized with 7% of MgCl2 mixed with seawater 1:1 and weighted. Subsequently, these were decapitated by cutting off the prostomium plus the first 6 or 10 segments, using surgical scissors. To find out the influence of the position of the cut along the anterior-posterior axis (A-P) axis, two different cuts were performed on small specimens reared at 22 °C: at the 10th segment (n = 3 worms per aquaria), which corresponds to the area at the end of the pharynx (Marsden 1963), and at the 6th segment (n = 3 worms per aquaria). Due to the paucity of the individuals, only the cut at the 10th segment was made in large specimens.
After amputation, the worms were observed and photographed once a week using a Nikon SMZ25 stereomicroscope fitted with NIS-Elements imaging software, and they were weighed once a month. The whole experiment lasted 24 weeks post amputation (24 weeks p.a.) until all worms regenerated a functionally complete anterior part.
At the end of the experiment, the worms were examined and dissected to verify the internal mouth formation, the sexual maturation status, and the lipid content.
A control group of non-amputated worms was also maintained in each thermostatic chamber (10 small and 10 large worms in each condition) as a reference condition for the experimental observations on sexual maturation and lipid content. To secure the comparability of results, the control worms were starved throughout the experiment to simulate the lack of food ingestion of the amputated specimens.
Finally, an additional large individual was accidentally broken into four parts during transportation to the laboratory: the anterior part with the original prostomium, two central parts lacking both the prostomium and pygidium, and the posterior part including the terminal pygidium. The four parts were kept isolated in a separate rearing aquarium at 22 °C throughout the amputation experiment.
Histological analysis
Ten specimens collected in November were examined before the amputation experiment, together with 3 small worms randomly taken from each tank (conditions and control) at the end of the experiment.
From each specimen, 3 pieces each consisting of two segments (central and posterior part of the body) were dehydrated using different concentrations of ethanol (80–100%), cleared with Xylene (histological grade), and impregnated with paraffin (Biooptica, melting point 56–58 °C). The tissue was then embedded in paraffin, sectioned at 7 μm with a Leica microtome (RM 2155), and stained with hematoxylin-eosin solution (SIGMA).
Lipid and organic matter determination
After the amputation, all the anterior parts of the SW (small worms reared at 22 °C) specimens were frozen at -80 °C for lipid extraction, alongside the completely regenerated worms, and lyophilized (-40 °C). Approximately ∼ 10 mg of each sample dry weight (DW) was reduced to ash in a muffle furnace (Relp 2 H-M9) for five hours at 450 °C. The percentage of organic matter (or ash-free dry weight-AFDW) was then calculated as the difference between the DW and the weight of the ash (Slattery and McClintock 1995). AFDW was used to normalize lipids data. The amount of lipids was quantified colorimetrically using ∼ 10 mg of DW (anterior segments). The tissue powder was resuspended in 3 mL of chloroform: methanol (2:1) (Barnes and Blackstock 1973; modified by Rossi et al. 2006). A calibration line was made using cholesterol as standard and the final reaction was performed using vanillin. Colorimetric measurements were read using a spectrophotometer (UV Mini1240, Shimadzu). Results are expressed in µg lipid mg− 1 AFDW. The same method was employed for the “control” specimens at 22 °C, to be compared with the completely regenerated worms. Results of weight, lipids concentration, and organic matter were analysed using an ANOVA test.
Results
General morphological changes
Shortly after amputation, as a starting point of anterior regeneration, all treated H. carunculata worms exhibited a shrinkage of the segment most proximal to the wound to seal the coelomic cavity. This contraction led the parapodia closest to the cut to bend forward and parallel to the main body axis. The process of wound healing was completed within 48 h (Fig. 1A). During the third-week post amputation (3 weeks p.a.), a regenerative blastema - visible as a whitish patch - appeared ventrally in 10 specimens (Fig. 1B). A week later, a reddish bud (i.e., the future prostomium) was observed between the two bent parapodia, providing evidence of the ongoing head regeneration (Fig. 1C). At 4 weeks p.a., two pairs of eyes and two palps early appeared in the prostomium bud (Fig. 1D). The novel prostomium was fully regenerated within the following 6–12 weeks, as the development of the palps, antennae, eyes, and caruncula was completed, along with the formation of three novel segments bearing parapods, chaetae, and branchial filaments (Fig. 1E, F). The regenerated organs and segments of the worms gradually increased their volume, and the full regeneration of the mouth parts allowed the worms to evert their pharynx 4 months p.a. (Fig. 1G, H).
Anterior regeneration in Hermodice carunculata. (A) 48 h post amputation. (B) Blastema formation 3 weeks p.a. (C) reddish bud (future prostomium) after 4 weeks p.a. (D) Early comparison of eyes and palps after 4 weeks p.a. (E) Formation of the first chaete and segments after seven weeks. (F) 12 weeks p.a. (G, H) Dorsal and ventral view after 4 months post amputation. (Bl. Blastema; Rb. Reddish bud; Pl. Palp; Ey. Eye/s; An. Antenna/ae; Mo. Mouth; Ch. Chaete; Bf. Branchial filaments; Cr. Caruncula; No. Notopodia; Ne. Neuropodia). Scale A, B 4 mm; C 1 mm; D 0.5 mm E, F 3 mm; G,H 2 mm
Developmental anomalies during the regenerative process were observed at both experimental temperatures (14 °C and 22 °C). Five specimens presented malformations of the branchial filaments (Fig. 2A). Two specimens missed the regeneration of the single antennae below the caruncula (Fig. 2B, C).
Difference in regeneration time
The timing of regeneration and survival varied across the two size classes and between the temperature treatments (Table 1).
All amputated worms maintained at 22 °C survived the amputation and completed the regenerative process, with all the anterior parts fully regenerated. The regeneration time was different for the small and large worms reared at 22 °C (SW, LW), with a faster regenerative process in the SW group. Specifically, the reddish bud formation occurred at 4 weeks p.a. in the SW group and 9 weeks p.a. in the LW group. The first regenerated segment was visible since 6 weeks p.a. and since 11 weeks p.a. in SW and LW groups, respectively. The observations on specimens belonging to the SW group were completed at 12 weeks p.a., due to the complete regeneration of the mouth, enabling the worms to eat. Conversely, in the LW group, the regenerative process was finalized at 20 weeks p.a. However, both SW and LW showed the same morphological patterns of anterior regeneration.
Experimental worms kept at 14 °C displayed 100% mortality at 24 weeks p.a. Before that, the regeneration of the reddish bud occurred at 14–20 weeks p.a. in the small sized worms reared at 14 °C (cold conditions, SC group). All large specimens at 14 °C (LC) died during the blastema formation, by 20 weeks p.a. small specimens reared at 22 °C (SW) with amputation at the 10th segment posterior to the prostomium (p.p.) regenerated up to 5 segments; whereas individual worms amputated at the 6th p.p. regenerated a maximum of 3 segments (Fig. 3). At the same time after amputation (12 weeks) (Fig. 3), the organisms in which 10 segments were cut off, regenerated between 5 and 6 segments (Fig. 3A, C) while when six segments were removed, only three segments were regenerated (Fig. 3B, D).
Bi-directional regeneration potential
The accidental fragmentation of an individual into four parts led to the formation of four individual worms, each showing the regeneration of missing parts. The anterior fragment, bearing the head, regenerated terminal posterior segments, including the pygidium. The two middle parts regenerated both anterior and posterior segments, including head and pygidium. The posterior fragment regenerated the most anterior segments, including head. The regeneration of the posterior segments and pygidium appeared faster than the regeneration of the anterior segments and head (Fig. 4).
H. carunculata under anterior and posterior differentiation. (A) Initial worm during posterior regeneration. (B,C,D) Prostomium of the three different pieces in sequence A-P axis. Ventral view of the three new worms; red circle represents anterior regeneration and the red line the start of the posterior regeneration. (F) Dorsal view of three new worms; the numbers represent the order of the cuts made relative to the initial worm. B, C, D 500 μm. A, E, F 1 cm
Sexual maturity
Histological analyses carried out on 10 worms at the timing of sampling (November) showed the absence of gametes in the coelomic cavities of all specimens, supporting the assumption that all experimental specimens were in a spent phase at the start of the experiment.
Examination of control specimens at 12 weeks from the start of the experiment revealed evidence of the different stages of gametogenesis in the same individual at both experimental temperatures in male and female worms (14 °C and 22 °C).
Undifferentiated male germ cells (spermatogonia) were observed at 12 weeks from the start of the experiment, surrounding blood vessels (Fig. 5A-C) while masses of early spermatids were found embedded in a botryoidal-like tissue (Kudenov 1974). The last stage of spermatogenesis (i.e., full differentiation of spermatozoa) was detected in the coelomic cavity.
Females showed the transversal body section (Fig. 5D-F) with different stages of gamete maturation in the same worm, within the botryoidal-like tissue, without the presence of well-defined ovaries at 12 weeks from the start of the experiment. Pre-vitellogenic oocytes were found; these were distinguished by a central nucleus and a small darker nucleolus. Mature oocytes of approximate size of 80–100 μm are characterized by a large germinal vesicle and a nucleolus.
Female control specimens at 22 C° showed rounded oocytes at a more advanced state of maturation compared to partially wrinkled oocytes detected in control worms reared at 14 °C. In the dissected regenerating individuals, the presence of male and female gametes was observed at both temperatures 12 weeks p.a. In LW at the 16th week a spawning event was observed in the aquaria (Fig. 5G).
(A,B,D,E) Histological analysis (transverse sections) of H.carunculata. (A, B) Male specimens. (C)Mature spermatozoids. (D, E) Female specimen. (F) Mature egg. (G) Female specimen during spawning. (Bt. Botryoidal-like tissue; St. Spermatogonia; Bv. Blood vessel; Mo. Mature oocytes; Po. Pre-vitellogenic oocytes). Scales: A, C 100 μm; B, D 50 μm; C, F 20 μm; G 1 cm
Lipid content, organic matter and body weight
As expected, all small specimens kept at 22 °C at 12 weeks p.a. showed a significant decrease in weight (F1,19= 26, p < 0.001), from 3.8 ± 0.6 (SD) g to 2.5 ± 0.5 (SD) g.
In contrast, the total lipid content of SW was significantly lower in the initial amputated anterior parts compared to the content of lipids measured in fully regenerated anterior parts (F1,19= 9.5, p < 0.01), shifting from 98.7 ± 13.2 (SD) µg lipids /mg AFDW to 117.7 ± 13.4 (SD) µg lipids/mg AFDW (Fig. 6). The concentration of lipids in the control was significantly different (F2,29= 56.3, p < 0.001), with a mean of 207.6 ± 39.7 (SD) µg lipids /mg AFDW. No significant difference (F2,29= 0.47; p > 0.05) was observed in the organic matter (OM) content which was 87% ± 7 (SD) ( 0.87 ± 0.07 g/g DW) and 84% ± 7 (SD) (0.84 ± 0.07 g/g DW) for the amputated head and regenerated body respectively, and 87% ± 9 (SD)( 0.87 ± 0.09 g/g DW) for the control.
Discussion
Overall, our results show that H. carunculata fireworms can regenerate amputated anterior segments and organs, including fully functional sensory structures, mouth opening, and pharynx. Laboratory experiments showed that regeneration occurred at both 14 °C and 22 °C, but they were significantly slower at 14 °C. Complete regeneration during the experimental period was observed only at 22 °C; this experimental evidence is consistent with the thermophilic nature of H. carunculata, native of the southernmost (and warmest) coastal areas of the Mediterranean Sea.
During the regenerative process at 22 ºC some malformations were also observed in some individuals. Commonly, the morphology of the head of H. carunculata includes median caruncle followed by a median antenna, two pairs of eyes, two antennae, and two palps. Malformations can occur at the level of median antenna, but also in the development of the branchial filaments, which were observed to collapse after 8 weeks, eventually resembling a small knob, before re-acquiring their typical morphological features by the time that the regenerative process is completed. By investigating the phenotypic plasticity of H.carunculata specimens collected from different locations worldwide, it was suggested the depletion of dissolved oxygen may locally drive an epigenetic adaptive response, with the development of a higher number of gill filaments (Ahrens et al. 2013; Grimes et al. 2020).
Although the initial regenerative patterns were similar across the different experimental conditions (14 °C vs. 22 °C, SW vs. LW), the regeneration process started but did not complete at 14 °C (all experimental worms died by 24 weeks p.a.). A difference of water temperature of 8 °C between the two experimental conditions (22 vs. 14 °C) suddenly produced a shift of ten weeks at the start of the regenerative blastema formation. A sharp warm-temperature affinity in H. carunculata is indirectly witnessed also by the effect of temperature on its predatory performance (measured as ingestion rate), which in laboratory experiments - using a preferred coral prey - was found maximal at 25,6 °C (Bosch-Belmar et al. 2024). A temperature-dependent regeneration rate was also found in other polychaetes, namely Sabella spallanzanii (Gmelin, 1791), Sabella pavonia (Savigny, 1822) and Diopatra neapolitana Delle Chiaje, 1841 (Berrill 1931; Licciano et al. 2012; Pires et al. 2015), showing enhanced regeneration at warmer temperatures.
Interestingly, Grimes et al. (2020) showed that hypoxia conditions reduce regeneration rates in H. carunculata, suggesting that increasing water temperatures - when coupled to a reduction in dissolved oxygen concentration in the water - might impair, and not enhance, the regenerative capability of fireworms. We suggest that H. carunculata requires warm water to continue the process, but that oxygen saturation should not fell below a minimal threshold to grant an optimal functioning of cellular mechanisms of regeneration. This might explain the anecdotic absence of fireworms from shallow coastal lagoons and sheltered areas, where, due to reduced water circulation and high temperatures, hypoxic conditions can be rapidly encountered. On this respect, new experiments with combinations of different water temperatures, oxygen concentrations, and food availability levels, will help to map the expected distribution of H. carunculata in the Mediterranean Sea under the predicted climatic crisis scenarios.
Small-size worms (∼ 4 g) showed a faster regeneration than large-sized worms (∼ 25 g). A comparable result was obtained by Planques et al. (2019) on Platynereis dumerilii (Audouin and Milne Edwards, 1833) posterior regeneration. The concurrent bidirectional regeneration at the same time in the same fragment was also observed, as most occurs in planarians (Saló and Baguña 2002). In the small-sized worms, the number of regenerated segments posterior to the new prostomium was lower than the amputated segments occurring in the original specimens. On average, the number of regenerated segments was half the number of the amputated segments, i.e., 3 regenerated segments were formed following a 6-segment amputation, and about 5–6 regenerated segments were formed following a 10-segment amputation.
Among annelids, several species exhibit the ability to regenerate the anterior end, with the extent of this capability varying according to the location of amputation (i.e., the number of removed segments) along the anterior-posterior axis (Kostyuchenko and Kozin 2021). In some species, a reduction of the regenerative potential is observed when more segments are removed (Nengwen et al. 2011). Taking into consideration the limitations of our experiment - only to two different amputation sites (6th or 10th segment posterior to prostomium) - preliminary experimental evidence suggests H. carunculata maintains a proportional segment regeneration potential along the body axis, at least to the 10th segment posterior to the prostomium.
As the regeneration process in annelids is initiated mainly by the involvement of a proliferative blastema, an equally distributed regeneration potential along the main body axis - from the anterior to the posterior body ends – might be secured by a local origin of the blastema near or at the site of the wound, with limited migration and involving cells from all the ectodermal, mesodermal and endodermal layers (Bely 2014). Further studies are needed to identify a unitary model of regeneration to understand the morphogenetic potential of local de-differentiation combined with a migratory pool of undifferentiated stem cells (as the neoblast cells identified in clitellates) (Zattara et al. 2016).
The regeneration rate can be affected by the individual stage of reproductive maturation, as the ability to regenerate missing parts appears reduced during the time of reproduction, because of the high energy investment for gametogenesis, impairing the ability of regeneration (Maginnis 2006). In regeneration experiments, a 100% mortality was observed in the sabellid Branchiomma luctuosum (Grube, 1870) during the spawning period (Licciano et al. 2012, 2015); similarly, a reduced rate of regeneration and survivorship was found in Myxicola infundibulum when worms were ripe (Prentiss et al. 2017). Conversely, H. carunculata seems not to follow this rule, since both small and large specimens can undergo gametogenesis and anterior regeneration at the same time. At the beginning of our experiments (November), histological analyses confirmed that the source population of experimental worms was in a spent phase; on the contrary, at the end of experiment the dissection of the worms maintained at the two different temperatures (14 °C and 22 °C) revealed that the gametogenic cycle was ongoing in both cases. In the field, H. carunculata reproduces in summer (Toso et al. 2020), with gametogenesis starting in May/June, and spawning occurring between July and September (80–100 days). The histological analysis of specimens maintained under laboratory conditions revealed the presence of different stages of gametogenesis, suggesting that this species can have multiple spawning events during the favourable season. The entire gametogenesis observed under laboratory conditions lasted 80–100 days, and all the worms at both temperatures (14 °C and 22 °C) showed the same patterns of gamete development after amputation and, therefore, there seems to be no energetic trade-off between sexual reproduction and regeneration in H. carunculata. However, although individuals reared at the low temperature (14 °C) produced gametes, their oocytes were not as well differentiated as they were in the specimens maintained at 22 °C. During the formation of gametes, a significant increase in lipid concentration (shifting from 98.7 ± 13.2 (SD) µg lipids/mg AFDW before amputation to 117.7 ± 13.4 (SD) µg lipids/mg AFDW after amputation) was observed in the SW during the regeneration time (12 weeks). The anabolic pathway of these molecules, reflecting a change in the metabolism of the animal, was around 16%. The same patterns in the concentration of lipids were detected in the worms of the control group, in which the lipids increased over the 12 weeks. Since these worms did not need to invest energy in the anterior regeneration, energies were employed for the production of gametes. As reported by Martinez (1991), the increase in lipid concentrations can be correlated to the production of gametes. Thus, these results seem to support the hypothesis that the high demand for energy needed for regeneration does not impair the production of gametes in H. carunculata.
Considering the basal position of the Amphinomidae in the annelid phylogeny, our observations on H. carunculata corroborate the hypothesis by Zattara and Bely (2016), who considered anterior and posterior regeneration as an ancestral feature of Annelida and a key developmental innovation for the evolution of fission as a mode of asexual reproduction, particularly appropriate for rapid expansion of individuals and the consequent exploitation of seasonal resources, available for a brief period. However, despite the strong regenerative capabilities of H. carunculata, in situ fission has not been observed in nature. Conversely, laboratory-based, repeated mechanical stimulation (by forceps) on the same body segment of healthy worms, may led the worms to initiate and carry out fission, as observed following injury and rupture of a single specimen into four fragments, all starting bidirectional regeneration (Fig. 4).
Altogether, these observations suggest that the wide potential of H. carunculata to repair physical damage can likely derive from adaptive redeployment of genes used during embryogenesis, tissue homeostasis, and reproduction to control cell differentiation. It can be hypothesized that the recruitment of bidirectional regenerative processes, as observed in H. carunculata, might be involved in driving adaptive architomic fission induced by environmental stressors, as observed during our experimental manipulations. Apparently, this potential does not support a genetically programmed asexual reproduction process in the H. carunculata life cycle. However, future investigations will be required to determine whether the bidirectional regeneration ability is fully retained by H. carunculata in the field; or whether these developmental controls can be upregulated - in the absence of wounds and injuries - to carry out programmed asexual reproduction by fission.
Fireworms are native invasive predators in many coastal areas of the Mediterranean Sea, where artisanal fishermen frequently encounter a significant quantity of H. carunculata as accidental bycatch in their nets (Celona and Comparetto 2010; Tiralongo et al. 2023), often preying on fish captured by their nets. Intentionally (to kill worms) and unintentionally, fishermen usually cut fireworms into multiple fragments and discard them returning the worm fragments into the sea (A. Toso, pers. comm). In light of the high regenerative potential of H.carunculata, this practice may inadvertently contribute to increase abundance and spreading of fireworms in coastal areas, as observed in the harvesting of Diopatra aciculata Knox and Cameron, 1971 (Schoeman and Simon 2023).
In conclusion, H. carunculata seems to adapt to high stress levels such as a partial loss of its body, being able not only to recover from injuries through regeneration but also continue the reproductive cycle during a period in which is not able to feed, maintaining its fitness and a constant population size. Moreover, the ability for anterior regeneration across different temperatures (without any apparent interference with sexual reproduction), along with the increasing abundance of fireworms observed during the last years (Toso et al. 2022), point out this species as a potential winner in the climate change scenario of higher seawater temperatures.
References
Ahrens JB, Borda E, Barroso R, Paiva PC, Campbell AM, Wolf A, Nugues MM, Rouse GW, Schulze A (2013) The curious case of Hermodice carunculata (Annelida: Amphinomidae): evidence for genetic homogeneity throughout the Atlantic Ocean and adjacent basins. Mol Ecol 22(8):2280–2229. https://doi.org/10.1111/mec.12263
Ahrens JB, Kudenov JD, Marshall CD, Schulze A (2014) Regeneration of posterior segments and terminal structures in the bearded fireworm, Hermodice carunculata (Annelida: Amphinomidae). J Morphol 275:1103–1112. https://doi.org/10.1002/jmor.20287
Barnes H, Blackstock J (1973) Estimation of lipids in marine animals and tissues: detailed investigation of the sulphophosphovanilun method for ‘total’ lipids. J Exp Mar Biol Ecol 12(1):103–118. https://doi.org/10.1016/0022-0981(73)90040-3
Barroso R, Almeida D, Contins M, Filgueiras D, Dias R (2016) Hermodice carunculata (Pallas, 1766) (Polychaeta: Amphinomidae) preying on starfishes. Mar Biodivers 46:333–334. https://doi.org/10.1007/s12526-015-0394-9
Bely AE (2006) Distribution of segment regeneration ability in the Annelida. Integr Comp Biol 46:508–518. https://doi.org/10.1093/icb/icj051
Bely AE (2010) Evolutionary loss of animal regeneration: pattern and process. Integr Comp Biol 50:515–527. https://doi.org/10.1093/icb/icq118
Bely AE (2014) Early events in Annelid Regeneration: a Cellular Perspective. Integr Comp Biol 54:688–699. https://doi.org/10.1093/icb/icu109
Bely AE, Nyberg KG (2010) Evolution of animal regeneration: re-emergence of a field. Trends Ecol Evol 25(3):161–170. https://doi.org/10.1016/j.tree.2009.08.005
Bely AE, Sikes JM (2010) Latent regeneration abilities persist following recent evolutionary loss in asexual annelids. Proc Natl Acad Sci 107:1464–1469. https://doi.org/10.1073/pnas.0907931107
Bely AE, Wray GA (2001) Evolution of regeneration and fission in annelids: insights from engrailed and orthodenticle class gene expression. Dev 128:2781–2791. https://doi.org/10.1242/dev.128.14.2781
Berrill NJ (1931) Regeneration in Sabella pavonine (sav.) And other sabellid worms. J Exp Zool 58:495–523. https://doi.org/10.1002/jez.1400580123
Bosch-Belmar M, Tantillo MF, Sarà G (2024) Impacts of increasing temperature due to global warming on key habitat-forming species in the Mediterranean Sea: unveiling negative biotic interactions. Glob Ecol Cons e02844. https://doi.org/10.1016/j.gecco.2024.e02844
Brockes JP, Kumar A (2008) Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol 24:525–549. https://doi.org/10.1146/annurev.cellbio.24.110707.175336
Celona A, Comparetto G (2010) Prime osservazioni sulla predazione opportunistica del vermocane Hermodice carunculata (Pallas, 1766), ai danni della piccola pesca artigianale nelle acque di Lampedusa (is. Pelagie). Annales Series Hist Nat. pp.15–20
Cosentino A, Giacobbe S (2011) The new potential invader Linopherus canariensis (Polychaeta: Amphinomidae) in a Mediterranean coastal lake: colonization dynamics and morphological remarks. Mar Pollut Bull 62(2):236–245. https://doi.org/10.1016/j.marpolbul.2010.11.006
David AA, Williams JD (2012) Asexual reproduction and anterior regeneration under high and low temperatures in the sponge associate Polydora colonia (Polychaeta: Spionidae). Invertebr Reprod Dev. 56(4), 315–324. https://doi.org/10.1080/07924259.2011.638404
De Vooys CGN (1975) Glycogen and total lipids in the lugworm (Arenicola marina) in relation to reproduction. J Sea Res 9(3–4):311–319. https://doi.org/10.1016/0077-7579(75)90006-X
Fishelson L (1971) Ecology and distribution of the benthic fauna in the shallow waters of the Red Sea. Mar Biol Int J Life Ocean Coast Waters 10(2):113–133. https://doi.org/10.1007/BF00354828
Franke HD (1999) Reproduction of Syllidae (Annelida: Polychaeta). Hydr 402:39–55. https://doi.org/10.1023/A:1003732307286
Fusco G, Minelli A (2023) Understanding Reproduction. Cambridge University Press. https://doi.org/10.1017/9781009225922
Gibson GD, Paterson IG (2003) Morphogenesis during sexual and asexual reproduction in Amphipolydora vestalis (Polychaeta: Spionidae). NZ J Mar Freshwater Res. 37:741 – 52. https://doi.org/10.1080/00288330.2003.9517204
Giese AC (1966) Lipids in the economy of marine invertebrates. Physiol Rev 46(2):244–298. https://doi.org/10.1152/physrev.1966.46.2.244
Grimes CJ, Paiva PC, Petersen LH, Schulze A (2020) Rapid plastic responses to chronic hypoxia in the bearded fireworm, Hermodice carunculata (Annelida: Amphinomidae). Mar Biol 167(9):1–10. https://doi.org/10.1007/s00227-020-03756-0
Kostyuchenko RP, Kozin VV (2020) Morphallaxis versus Epimorphosis? Cellular and Molecular aspects of Regeneration and Asexual Reproduction in Annelids. Biol Bull Russ Acad Sci 47:237–246. https://doi.org/10.1134/S1062359020030048
Kostyuchenko RP, Kozin VV (2021) Comparative aspects of Annelid regeneration: towards understanding the mechanisms of Regeneration. Genes 12:1148. https://doi.org/10.3390/genes12081148
Krželj M, Cerrano C, Di Gioia C (2020) Enhancing diversity knowledge through Marine citizen science and social platforms: the case of Hermodice carunculata (Annelida, Polychaeta). Divers 12(8). https://doi.org/10.3390/d12080311
Kudenov JD (1974) The reproductive biology of Eurythoe complanata (Pallas, 1766). America, 204
Langeneck J, Del Pasqua M, Licciano M, Giangrande A, Musco L (2020) Atypical reproduction in a syllid worm: the stolon of Syllis Rosea (Annelida, Syllidae) takes care of its offspring. J Mar Biol Assoc UK 100(2):221–227. https://doi.org/10.1017/S0025315420000119
Lewis J, Crooks R (1996) Foraging cycles of the amphinomid polychaete Hermodice carunculata preying on the calcereous hydrozoan Millepora complanata. Bull Mar Sci 58:853–856
Licciano M, Murray JM, Watson GJ, Giangrande A (2012) Morphological comparison of the regeneration process in Sabella spallanzanii and Branchiomma Luctuosum. Invertebr Biol 131:40–51. https://doi.org/10.1111/j.1744-7410.2012.00257.x. Annelida, Sabellida
Licciano M, Watson GJ, Murray JM, Giangrande A (2015) Evidence of regenerative ability in Myxicola infundibulum (Annelida, Sabellida): evolutionary and systematic implications. Invertebr Biol 134:48–60. https://doi.org/10.1111/ivb.12077
Lucey NM, Collins M, Collin R (2020) Oxygen-mediated plasticity confers hypoxia tolerance in a corallivorous polychaete. Ecol Evol 10(3):1145–1157. https://doi.org/10.1002/ece3.5929
Maginnis TL (2006) The costs of autotomy and regeneration in animals: a review and framework for future research. Behav Ecol 17:857–872. https://doi.org/10.1093/beheco/arl010
Marsden JR (1963) The digestive tract of Hermodice Carunculata (Pallas). Polychaeta: Amphinomidae. Can J Zool 41:165–184. https://doi.org/10.1139/z63-020
Martinez G (1991) Seasonal variation in biochemical composition of three size classes of the Chilean scallop Argopecten Purpuratus Lamarck, 1819. Veliger 34:335–343
Morris RJ, Sargent JR (1973) Studies on the lipid metabolism of some oceanic crustaceans. Mar Biol 22(1):77–83. https://doi.org/10.1007/BF00388913
Müller MCM, Berenzen A, Westheide W (2003) Experiments on anterior regeneration in Eurythoe complanata (Polychaeta, Amphinomidae): reconfiguration of the nervous system and its function for regeneration. Zoomorphol 122:95–103. https://doi.org/10.1007/s00435-003-0073-4
Nakamura K, Tachikawa Y, Kitamura M, Ohno O, Suganuma M, Uemura D (2008) Complanine, an inflammation-inducing substance isolated from the marine fireworm Eurythoe complanata. Org Biomol Chem 6:2058–2060. https://doi.org/10.1039/B803107J
Nengwen X, Feng G, Edwards CA (2011) The regeneration capacity of an earthworm, Eisenia fetida, in relation to the site of amputation along the body. Acta Ecol Sinica 31:197–204. https://doi.org/10.1016/j.chnaes.2011.04.004
Pires A, Figueira E, Moreira A, Soares AMVM, Freitas R (2015) The effects of water acidification, temperature, and salinity on the regenerative capacity of the polychaete Diopatra neapolitana. Mar Environ Res 106:30–41. https://doi.org/10.1016/j.marenvres.2015.03.002
Planques A, Malem J, Parapar J, Vervoort M, Gazave E (2019) Morphological, cellular and molecular characterization of posterior regeneration in the marine annelid Platynereis dumerilii. Dev Biol 445(2):189–210. https://doi.org/10.1016/j.ydbio.2018.11.004
Prentiss NK, Tyler MS, Dean D (2017) A morphological and histological investigation of the regeneration in Myxicola infundibulum (Montagu, 1808) (Sabellida, Annelida). J Mar Biol Assoc UK 97:1155–1165. https://doi.org/10.1017/S0025315417000248
Ribeiro RP, Bleidorn C, Aguado MT (2018) Regeneration mechanisms in Syllidae (Annelida). Regeneration 5(1):26–42. https://doi.org/10.1002/reg2.98
Righi S, Maletti I, Maltagliati F, Castelli A, Barbieri M, Fai S, Prevedelli D, Simonini R (2019) Morphometric and molecular characterization of an expanding Ionian population of the fireworm Hermodice carunculata (Annelida). J Mar Biol Assoc UK 99(7):1–9. https://doi.org/10.1017/S002531541900064X
Righi S, Prevedelli D, Simonini R (2020) Ecology, distribution and expansion of a Mediterranean native invader, the fireworm Hermodice carunculata (Annelida). Mediterr Mar Sci 21(3):558–574. https://doi.org/10.12681/MMS.23117
Righi S, Forti L, Simonini R, Ferrari V, Prevedelli D, Mucci A (2022) Novel natural compounds and their anatomical distribution in the stinging Fireworm Hermodice carunculata (Annelida). Mar Drugs 20(9):585. https://doi.org/10.3390/md20090585
Rossi S, Gili JM, Coma R, Linares C, Gori A, Vert N (2006) Temporal variation in protein, carbohydrate, and lipid concentrations in Paramuricea clavata (Anthozoa, Octocorallia): evidence for summer-autumn feeding constraints. Mar Biol 149:643–651. https://doi.org/10.1007/s00227-005-0229-5
Rossi S, Coppari M, Viladrich N (2017) Benthic-Pelagic coupling: new perspectives in the animal forests. Marine Animal forests: the ecology of benthic biodiversity hotspots. Springer, pp 855–886. https://doi.org/10.1007/978-3-319-21012-4_23
Saló E, Baguñà J (2002) Regeneration in planarians and other worms: new findings, new tools, and new perspectives. J Exp Zool 292:528–539. https://doi.org/10.1002/jez.90001
Sargent JR (1995) Origins and functions of egg lipids: nutritional implications. In: Bromage NR, Roberts RJ (eds.), Broodstock Management and Egg and Larval Quality. Blackwell Sci Oxford. p 353
Schoeman S, Simon CA (2023) Live to die another day: regeneration in Diopatra Aciculata Knox and Cameron, 1971 (Annelida: Onuphidae) collected as bait in Knysna Estuary. S Afr Biol 12(3):483. https://doi.org/10.3390/biology12030483
Schulze A, Grimes CJ, Rudek TE (2017) Tough, armed and omnivorous: Hermodice carunculata (Annelida: Amphinomidae) is prepared for ecological challenges. J Mar Biol Assoc UK 97(5):1075–1080. https://doi.org/10.1017/S0025315417000091
Simonini R, Righi S, Maletti I, Fai S, Prevedelli D (2017) Bearded versus thorny: the fireworm Hermodice carunculata preys on the sea urchin Paracentrotus lividus. Ecol 98:2730–2732. https://doi.org/10.1002/ecy.1919
Simonini R, Maletti I, Righi S, Fai S, Prevedelli D (2018) Laboratory observations on predator–prey interactions between the bearded fireworm (Hermodice carunculata) and Mediterranean benthic invertebrates. Mar Freshw Behav Physiol 51(3):145–158. https://doi.org/10.1080/10236244.2018.1502031
Slattery M, McClintock JB (1995) Population structure and feeding deterrence in three shallow-water antarctic soft corals. Mar Biol 122:461–470. https://doi.org/10.1007/BF00350880
Tiralongo F, Marino S, Ignoto S, Martellucci R, Lombardo BM, Mancini E, Scacco U (2023) Impact of Hermodice carunculata (Pallas, 1766) (Polychaeta: Amphinomidae) on artisanal fishery: a case study from the Mediterranean Sea. Mar Environ Res 192:106227. https://doi.org/10.1016/j.marenvres.2023.106227
Toso A, Boulamail S, Lago N, Pierri C, Piraino S, Giangrande A (2020) First description of early developmental stages of the native invasive fireworm Hermodice carunculata (Annelida, Amphinomidae): a cue to the warming of the Mediterranean Sea. Mediterr Mar Sci 21(2):442–447. https://doi.org/10.12681/mms.22043
Toso A, Furfaro G, Fai S, Giangrande A, Piraino S (2022) A sea of fireworms? New insights on ecology and seasonal density of Hermodice carunculata (Pallas, 1766) (Annelida) in the Ionian Sea (SE Italy). Eur Zool J 89(1):1104–1114. https://doi.org/10.1080/24750263.2022.2113156
Weidhase M, Bleidorn C, Beckers P, Helm C (2016) Myoanatomy and anterior muscle regeneration of the fireworm Eurythoe cf. complanata (Annelida: Amphinomidae). J Morphol 277:306–315. https://doi.org/10.1002/jmor.20496
Weigert A, Bleidorn C (2016) Current status of annelid phylogeny. Org Divers Evol 16:345–362. https://doi.org/10.1007/s13127-016-0265-7
Yáñez-Rivera B, Méndez N (2014) Regeneration in the stinging fireworm Eurythoe (Annelida): lipid and triglyceride evaluation. J Exp Mar Bio Ecol 459:137–143. https://doi.org/10.1016/j.jembe.2014.05.023
Zattara EE, Bely AE (2016) Phylogenetic distribution of regeneration and asexual reproduction in Annelida: regeneration is ancestral, and fission evolves in regenerative clades. Invertebr Biol 135:400–414. https://doi.org/10.1111/ivb.12151
Zattara EE, Turlington KW, Bely AE (2016) Long-term time-lapse live imaging reveals extensive cell migration during annelid regeneration. BMC Dev Biol 16:6. https://doi.org/10.1186/s12861-016-0104-2
Zoran MJ (2010) Regeneration in Annelids. Encycl Lifi Sci Els. https://doi.org/10.1002/9780470015902.a0022103
Acknowledgements
The authors acknowledge the use of the excellent research facilities of the BIOforIU-Experimental Centre for Biodiversity and Ecosystem Research at Di.S.Te.B.A (University of Salento, Lecce), and thank prof. Luigi Musco, (University of Salento, Lecce) for critically reviewing this manuscript.
Funding
Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4 - Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union-NextGenerationEU. EU Horizon Euro Research and innovation program ACTNOW (grant agreement No: 101060072.
Open access funding provided by Università del Salento within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
A.T. study conception and design, material experimental preparation, field sampling, maintenance of the aquaria, histological analysis, lipids extraction, data collection original draft preparation; M.M. experimental support, lipids extraction, histological analysis original draft preparation; S.R. review and editing the draft; A.G. experimental design support, review and editing the original draft; S.P. experimental design support, writing review and editing the original draft.
Corresponding author
Ethics declarations
Awards number
Project code CN_00000033, Concession Decree No. 1034. Of 14 June 2022 adopted by the Italian Ministery of University and research, CUP D33C22000960007, Project title “ National Biodiversity Future Center- NBFC”.
Conflict of interest
the authors do not have any competing financial or non-financial interests to declar.
Ethical approval
all applicable international, national, and/or institutional guidelines for the care, use and collection of animals were followed.
Additional information
Communicated by P. Ramey-Balci .
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toso, A., Mammone, M., Rossi, S. et al. Effect of temperature and body size on anterior and posterior regeneration in Hermodice carunculata (Polychaeta, Amphinomidae). Mar Biol 171, 152 (2024). https://doi.org/10.1007/s00227-024-04468-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04468-5