Abstract
Body colouration, a trait under strong selection, is influenced by the visual background of the environment. The stable influence of depth on visual background dynamics is due to light attenuation along the water column. Depth is also a key factor driving diversification in Sebastes rockfishes, influencing variations in several biological traits. Comparisons between closely related species suggest that brightly coloured species (red, orange, or yellow) tend to inhabit deeper waters and have slower growth rates compared to their shallow-water counterparts with dominance of dark colours (black, brown, or grey). Here, we used 377 photos from 100 Sebastes species, along with recently developed methods of colour quantification and phylogenetic comparative analyses, to assess this trend. Our analyses confirmed the separation of body colouration regarding depth, which was accompanied by differences in growth rates and morphological traits. This indicated that variations in body colourations are included in the ongoing correlational selection process dictated by depth. Analyses of closely related species indicated that depth is an initial driver of colour differentiation and that colour differences do not progressively increase with genetic divergences. We hypothesized that the bright-coloured rockfishes are found in deeper waters because in shallow environments they are more vulnerable to the potential negative effects of UV radiation and higher predation risk, while their predation successes are lessened, in comparison to dark-coloured rockfishes. Overall, this study emphasizes the intricate relationship between genetics, environment, adaptation, and the striking diversity of body colourations observed in Sebastes rockfishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Body colouration strongly dictates how species interact within their respective environments because body colouration is a trait under strong, selective pressures. In environments where the visual background is influenced by a combination of factors, social signalling, effectiveness of camouflage, reproductive success, and fitness for a given species can vary substantially because of its body colouration (Gray and McKinnon 2007; Kekäläinen et al. 2010; Lehtonen et al. 2023). Factors contributing to the complexity of the visual background in marine environments include turbidity, depth, type of substrate, presence of prey, predators, and other species such as corals, seaweeds, and seagrass beds, which serve as hideouts (Marshall et al. 2019; Salis et al. 2019; dos Anjos et al. 2022). Together, these elements create a dynamic backdrop against which a species must adapt. However, one clear aspect of this interplay of environmental variables is the consistent light attenuation along the depth gradient, which serves as a stable environmental axis that can exert substantial influence on the diversification of body colouration of species.
The genus Sebastes Cuvier 1829 is a diverse group encompassing over 110 species that are colloquially called rockfishes (Hyde and Vetter 2007; Ingram and Kai 2014). These species can be found inhabiting different marine environments, from shallow coastal waters to profound depths of over 1000 m. Many Sebastes species, roughly 70 of them, are found in the Northeast Pacific Ocean, whereas the northwestern Pacific Ocean is home to approximately 30 different species (Hyde and Vetter 2007; Ingram and Kai 2014). Within the genus, recently diverged sister species show wide overlap in latitudinal range, but less overlap in depth distribution (Ingram 2011). Extensive research and phylogenetic comparative analyses indicate that the depth at which rockfishes reside is a primary driver of diversification, leading to the development of significant differences in body shape, eye size, otolith shape and size, acoustic and visual sensitivity, and lifespan (Stefánsson et al. 2009; Ingram and Shurin 2009; Sivasundar and Palumbi 2010; Ingram 2011, 2015; Shum et al. 2014; Ingram and Kai 2014; Tuset et al. 2015, 2016; Heras and Aguilar 2019; Kolora et al. 2021; Treaster et al. 2023). The combination of certain values in these biological traits suggests the occurrence of correlational selection, in which multiple traits are simultaneously influenced by a single ecological constraint (Lande and Arnold 1983). In several groups, this type of selection is usually accompanied by colouration differences (McKinnon and Pierotti 2010), offering valuable insights into the process of speciation in parapatry and sympatry (Gray and McKinnon 2007; Forsman et al. 2008; McLean and Stuart-Fox 2014).
Rockfishes undergoing speciation can differ in body colourations in concordance with asymmetric depth distributions within their habitats (Hyde et al. 2008; Venerus et al. 2013; Artamonova et al. 2013; Zhuang et al. 2015, 2020; Deville et al. 2023a), and adaptive changes in visual perception (Sivasundar and Palumbi 2010; Shum et al. 2014; Deville et al. 2023b). Colour differences between fishes can be attributed to differences in the synthesis pathways of chromatophores and pigments, and the metabolism of acquired pigments through diet (e.g. Yamanome et al. 2007; Kumbar and Ganesh 2021; Fang et al. 2022). Both processes can be physiologically and morphologically regulated in response to the amount of light present in the visual surroundings of a species (Gamble et al. 2003; Pandolfi et al. 2003; Lehtonen et al. 2023). As a result, these environmentally influenced regulations in colouration may substantially contribute to the colour diversification of rockfishes, which dwell in various visual environments and experience rapid diversification.
The stunning diversity of colours across rockfishes varies from striking and vibrant hues of red, orange, and yellow to the more understated and cryptic shades of black, brown, and grey (Orr et al. 2000). Some species exhibit consistent colourations throughout their bodies, while others have unique colouration patterns characterised by the dominance of bright colours with irregular dark patches or spots, or vice versa, species with dominance of dark colours accompanied with bright patches or spots (Figs. 1 and 2). Ecomorphological comparisons of closely related species and ecotypes indicate that brightly coloured rockfishes tend to migrate into deeper areas during mating periods and consequently have slower growth rates than dark-coloured species (Venerus et al. 2013; Zhuang et al. 2015, 2020; Deville et al. 2023a). The depth segregation of rockfishes based on colourations may be possibly dictated by the need for photoprotective pigmentation in shallower waters. This is because the pigments responsible for bright colourations do not absorb UV irradiation as those producing dark colourations (Armstrong et al. 2000; Mizusawa et al. 2018). However, the depth segregation of bright- and dark-coloured rockfishes has not yet been comprehensively tested using quantitative comparative analyses and information from most species of the genus. The absence of these analyses may be attributed to the lack of appropriate image-analysis techniques and the limited availability of high-quality photographs. Recently developed methods that consider both the amount and similarity of colours (e.g. Van Belleghem et al. 2018; Weller and Westneat 2019) now allow us to quantify and compare the colouration of these species from digital photographs available in public repositories. In this study, we employed phylogenetic comparative analyses to evaluate the colour variation in Sebastes rockfishes considering (1) depth distribution of species, (2) life-history traits (growth rate), and biological characteristics that are also influenced by depth (e.g. eye width, body depth, etc., Ingram 2011; Ingram and Kai 2014), and (3) closely related species, particularly species pairs and triads categorised as species complexes (e.g. Roques et al. 1999; Kai and Nakabo 2004, 2008, 2013; Narum et al. 2004; Hyde and Vetter 2007; Hyde et al. 2008; Wilson et al. 2008; Muto et al. 2019; Nishimoto et al. 2019). We discuss how colourations, life-history traits, and morphological features of Sebastes rockfishes have been influenced by the respective habitats in which they live.
Colour categories in three rockfishes (S. aleutianus, S. babcocki, and S. nebulosus). Eight colour categories for these rockfishes were extracted from bodies excluding eyes, and dorsal, anal, and pelvic fins (shown within blue). Colour categories are represented in Hex codes and percentages of pixels
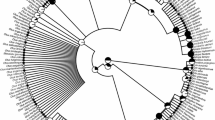
Mean four colour categories for 99 Sebastes rockfishes. Stacked barplots display colour categories in descending order from branches to tips of the phylogenetic tree. The three dotted circles around the barplots indicate the 30, 60, and 90% cumulative presence of the mean four colour categories. Photos of 16 species were included as references. Branches were coloured based on the geographical distribution of species found in FishBase. The phylogenetic tree with stacked barplots was plotted using the phytools package (Revell 2024)
Materials and methods
Data collection
A total of 377 photos from 100 of the 111 described Sebastes species were retrieved from iNaturalist, zukan, anglers, Flickr, Mexican-fish, WDFW (Washington Department of Fish and Wildlife), FishPix, and other online repositories (Supplementary Information Table S1). In the first-four repositories, the species identity of the photos is assigned and discussed by several contributors. In contrast, in Mexican-fish and WDFW, species identity is independently assigned by the repositories themselves, often uploading photos of reference individuals for each fish species. In the case of FishPix, species identity of photos is supported by the Kanagawa Prefectural Museum of Natural History and the National Museum of Nature and Science of Japan. For each species, photos with individuals displaying the colouration described in FishBase (Froese and Pauly 2023) and taxonomic descriptions were selected (Supplementary Information Table S2), leaving out descriptions with uncommon colourations (e.g. Muto et al. 2013a, 2016; Myoung et al. 2022). Photos of fresh specimens in lateral view and with clear illumination were chosen to reduce bias when detecting colours. At least three photos were compiled for 91 species, two for seven species, and one for two species. The average number of photos per species was 3.7. The dorsal, anal, and pectoral fins, and eyes were manually excluded from photos (Fig. 1, within blue). These fins were excluded because they were not extended in many photos, or they were partially covered by the pectoral fin or objects. Eyes were excluded because some individuals exhibited exophthalmia, causing abnormal whitish colourations that were not observed in other fishes. In species with fin colours significantly differing from body colours, the colours from the excluded fins are accounted for the pectoral and caudal fins. The backgrounds of the photos were removed, and photos were saved in PNG format for colour detection.
Data on the depth distribution ranges and growth rates of 97 and 77 species, respectively, were retrieved from FishBase, individual publications, and online repositories (Supplementary Information Table S3). The mean growth rate was considered for species with multiple entries in FishBase, whereas the combined growth rates of males and females were selected from individual publications. In seven species without growth-rate information, these rates were estimated from the maximum age using the formula of Froese (2022). For these estimations, the maximum age was attained and reported to be approximately 99% of the maximum body size and not 95%, as suggested by the author. A maximum body size of 99% was considered because, at 95%, the growth rates were underestimated by a factor of two in species with reported growth rates. In addition, data on the body depth, eye and pectoral fin widths, and lower jaw and gill raker lengths were retrieved for 91 species. These variables were selected because their variations are correlated with the depth distribution of species (Ingram 2011; Ingram and Kai 2014). Information on these morphological traits was compiled from Ingram and Kai (2014) and log-transformed for further analyses.
For phylogenetic reconstruction, we retrieved sequences of nine genetic regions (12S ribosomal RNA [12S], 16S ribosomal RNA [16S], cytochrome oxidase b [CytB], cytochrome oxidase c subunit I [COXI], mitochondrial control region [D-loop], proline transfer RNA [tRNA Pro], threonine transfer RNA [tRNA Thr], internal transcribed spacer 1 [ITS1], and the recombination activating 2 [RAG2]) from 108 species and four outgroups: Helicolenus avius, Hozukius emblemarius, Sebasticus marmoratus, and Sebastolobus alascanus. The genetic regions and outgroups were chosen from the following Hyde and Vetter (2007) and Ingram and Kai (2014). We included new sequences for S. cheni and S. ventricosus (16S, CytB, COXI, D-loop, tRNA Pro, tRNA Thr, and RAG2) (Supplementary Information Table S3). For species with multiple available sequences for one genetic region, the longest sequence was selected. Only S. diaconus and S. varispinis were left out because of the lack of DNA sequences.
Colour detection
Analyses were performed using R v.4.1.2 (R Core Team 2021). The getHistList function of the colordistance package (Weller and Westneat 2019) was used to decompose 10,000 pixels in each photo to values of red, green, and blue (RGB values) and binarize them into eight colour categories. The default binning method (histogram binning) was used because the colour categories have the same extensions for each photo, making comparisons of categories across photos simple. This method can also retain the details of photos, such as small colours, instead of collapsing them into a larger colour category (Weller and Westneat 2019). The output for each photo was a data frame containing centres of the eight colour categories in the RGB space, and the proportion of pixels contained within them. Colour categories of each photo were transformed into colour Hex codes for their use in plots.
Phylogenetic reconstruction
Each genetic region was separately aligned and then alignments were processed with Gblocks 0.9.1 (Castresana 2000) to discard poorly aligned positions while allowing the inclusion of gaps to increase the number of phylogenetically informative variable sites. The processed alignments were concatenated to obtain a final alignment of 5119 bp. Then, time-calibrated phylogenetic reconstruction was performed using BEAST v.2.6 (Bouckaert et al. 2019) following the instructions of Ingram and Kai (2014). For this reconstruction, the best substitution model from BEAST model test (Bouckaert and Drummond 2017) under all reversible model search, the Yule process to build the prior tree, and a fast relaxed Lognormal clock (Zhang and Drummond 2020), were used. The analysis was run using a Markov Chain Monte Carlo simulation of 150,000,000 steps, storing 10,000 trees from the posterior distribution. The maximum clade credibility tree (MCC tree) was calculated considering a 10% of burn-in with the programme TreeAnnotator included in BEAST v.2.6.
Colour variation across species
The RGB values and percentages of the eight colour categories extracted from each photo were used to estimate a matrix of pairwise Earth mover’s distances (EMD) with the getColorDistanceMatrix function. EMD were selected because they account for both similarity and amount of the colour categories to produce distance measurements for any pair of images (Weller and Westneat 2019). A total of 29 species pairs composed of sister species were identified based on previous phylogenetic trees (Hyde and Vetter 2007; Ingram and Kai 2014; Ingram 2015) and our estimated MCC tree (Fig. 2 and Supplementary Information Fig. S1). In 17 of these species pairs, the next closely related species (hereafter third species) was added to form species triads containing three species with sympatric occurrence (Supplementary Information Fig. S1). The matrix of pairwise EMD was partitioned into intraspecific EMD (hereafter “within” set), EMD between sister species (“sister” set), EMD of each species within a pair with the third species of a triad (“triad” set), and the rest of EMD between species (“other” set). To evaluate the differences between these four sets of EMD, box plots and an ANOVA test were constructed and performed, respectively.
For 98 species with more than one photo, the RGB values and percentages of the resulting eight colour categories were averaged to obtain consensus colour categories. For this purpose, data frames within each species were arranged by placing colour categories in descending order of presence. In 20 species, the descending orders of percentages from visually similar colour categories were consistent amongst individuals, while in other 78 species, the colour categories were manually arranged before estimating the consensus colour categories. By arranging them, we avoided homogenization and creation of highly dissimilar colour categories within each species. The eight consensus colour categories from each species were transformed into RGB integers using the formula (red × 2562) + (green × 256) + blue (Love et al. 2006).
A second matrix of EMD was estimated using the consensus colour categories of the species. This matrix of EMD was reduced using multidimensional scaling (hereafter PCoA, since it is also known as Principal Coordinate Analysis) (cmdscale function) to represent variation of colour categories amongst species. PCoA was used because this method projects high-dimensional data into a few dimensions while preserving the distances between observations. Correlations between the three axes of major variation (PCoA1, PCoA2, and PCoA3) and the RGB integers and percentages of the consensus colour categories of all species were assessed.
Phylogenetic comparative analyses
A phylomorphospace was constructed by projecting the MCC tree into a space of morphological traits defined by the PCoA axes (Sidlauskas 2008). Phylogenetic signals of the PCoA axes were estimated by considering the Pagel’s λ estimator (Pagel 1999). λ is a scaling parameter for the correlations between species with respect to the expected correlation under Brownian evolution. Then, phylogenetic generalized least squares (PGLS) were fitted to assess whether the PCoA axes were correlated with square-rooted values of the maximum depth distribution and growth rate of species while considering their phylogenetic relatedness. In addition, PGLS models were used to evaluate whether the PCoA axes are correlated with log-transformed values of body depth, eye and pectoral fin widths, and lower jaw and gill raker lengths. The possible influence of the phylogenetic signal on each interaction of variables was assessed by comparing a model with the estimated λ to a model with no phylogenetic signal (λ = 0).
Groups of species pairs and triads were used to assess the relationship between EMD and dissimilarities in depth distribution and phylogenetic distances while excluding earlier speciation events. Dissimilarity in depth distribution was estimated as the ratio of the differences in the minimum and maximum limits of the depth distribution of two species to the combined depth distribution of both species. Phylogenetic distances between species were estimated using the cophenetic.phylo function (ape package, Paradis and Schliep 2019). As each species triad contained three species (Supplementary Information Fig. S1), EMD, dissimilarities in depth distribution, and phylogenetic distances from each pair of species were added to obtain unique values for each triad. The tips of the phylogenies used for these analyses represent the comparisons between species pairs and triads; thus, the tips of the MCC tree were pruned to represent one member of each species pair and the third species of each species triad, respectively (Supplementary Fig. S1). PGLS models were carried out using these variables and the pruned MCC trees, as detailed in previous analyses.
Results
Within species, similar colour categories were observed across photos, despite differences in illumination and quality (Supplementary Information Fig. S2). More than 93% of the pixels were represented by four colour categories in all photos (Fig. 1 and Supplementary Information Fig. S3). The colour categories with the highest presence (hereinafter dominant colour categories) contained more than 50% of the pixels in 239 photos (Supplementary Information Fig. S3). Significant differences were found between the four sets of EMD (P value < 0.0001) except when the “sister” and “triad” sets were compared (P value > 0.50). The “within” set had a lower mean (0.1833 ± 0.007) than the “sister” set (0.2765 ± 0.0122), the “triad” set (0.2882 ± 0.0106), and the “other” set (0.3228 ± 0.0009) (Fig. 3). This indicates that it is possible to distinguish intraspecific and interspecific differences within the genus, allowing the analysis of colour variation between closely related species.
The dominant colour categories harboured more than 50% of the pixels in 65 species (Fig. 2). In the Northwest Pacific, 14 species of 29 exhibited dominant dark-colour categories, while the Northeast Pacific harbours 34 species (out of 57) with a dominance of bright colour categories (Fig. 2). The first three PCoA axes accounted for 51.34% of the variation. PCoA1 was negatively correlated with the RGB integers of the first and second most abundant colour categories (Table 1). Species with dominant colour categories related to black had positive values on the PCoA1 axis, while species with dominant colour categories in the ranges of red and orange had negative values on the same axis (Fig. 4). PCoA2 was positively and negatively correlated with the percentages of the first and second most abundant colour categories, respectively (Table 1). Species with large PCoA2 values exhibited high dominance in the first colour category (e.g. S. miniatus, S. phillipsi, S. ventricosus, and S. viviparus), whilst species with low PCoA2 values displayed two colour categories with similar percentages as observed in species with large, coloured patches (e.g. S. elongatus, S. owstoni, and S. serranoides) (Fig. 4 and Supplementary Information Fig S4). PCoA3 was not analysed or discussed, as it only accounted for 4.32% of the explained variation and its correlations with the RGB integers, and percentages of pixels were already accounted for PCoA1 and PCoA2 (Table 1).
Phylomorphospace of 100 species. Sky-blue circle indicates the confidence ellipse at 90% level. The phylomorphospace was constructed from the time-calibrated maximum clade credibility tree. Species were coloured based on the colour category with the highest presence. Symbols indicate geographic distribution of species. NAT North Atlantic, NEP Northeast Pacific, NWP Northwest Pacific, and TP Trans-Pacific. The relationship between S. diaconus and S. mystinus was represented as a dotted red line because of the lack of DNA sequences from S. diaconus. Some species with large values in extremes of the PCoA1 and PCoA2 axes were labelled. The scatter plot of explained variation for the first ten PCoA axes is displayed
The phylomorphospace showed convergences of dominant colour categories amongst distantly related species and some closely related species (Fig. 4). Only PCoA1 had a significant phylogenetic signal (λ = 0.6698, P value < 0.0001) under Brownian motion expectations. In contrast, the phylogenetic signal of PCoA2 indicated that variation within this axis is not significantly determined by the intrinsic phylogenetic relatedness of species (λ = 0.2007, P value > 0.15). Both the maximum depth distribution and growth rate were significant predictors of PCoA1 (maximum depth distribution: P value < 0.002, growth rate: P value < 0.023) in contrast to PCoA2, which was only correlated with growth rate (P value < 0.004) (Table 2). Scatter plots of PCoA1 on the maximum depth distribution and growth rate showed that species with dominance of bright colour categories are found in deeper waters and have lower growth rates than species with dominance of dark-colour categories, irrespective of their geographical distribution (Fig. 5). The scatter plots of PCoA2 on growth rate showed a decreasing trend between these variables (Fig. 5). PGLS models using information of species pairs only indicated a positive correlation between EMD and depth range dissimilarity (slope = 0.2023, P value < 0.05) (Fig. 6). PGLS models including species triads did not show any significant correlation between EMD and phylogenetic distance or depth range dissimilarity.
Phylogenetic generalized least squares (PGLS) models of PCoA1 and PCoA2 on square-rooted maximum depth distribution and growth rate. Species were coloured using their respective dominant colour categories. Symbols indicate geographical distribution of species. NAT North Atlantic, NEP Northeast Pacific, NWP Northwest Pacific, and TP Trans-Pacific. The only nonsignificant correlation displayed here is between PCoA2 and square-rooted maximum depth distribution. The regression lines and confidence intervals estimated using the PGLS models are represented by dotted lines and sky-blue areas, respectively
Both PCoA1 and PCoA2 were not correlated with body depth. PCoA1 was significantly correlated with both eye and pectoral fin widths (P values < 0.001), while PCoA2 was significantly correlated with lower jaw length (P value < 0.006) and eye width (P value < 0.02) (Table 2, Fig. 7). Gill raker length was significantly correlated with PCoA1 (P value < 0.001), but the correlation was lost after phylogenetic correction. The scatter plots of both PCoA1 and PCoA2 on the morphological traits indicated that species with dominance of bright colour categories had wider eyes and narrower pectoral fins than species with dominance of dark-colour categories (Fig. 7). Meanwhile species with uniform body colourations are endowed with wider eyes and larger lower jaws than species with large, coloured patches.
Phylogenetic generalized least squares (PGLS) models of PCoA1 and PCoA2 on morphological traits. Morphological traits were log-transformed for these analyses. Species were coloured based on the colour with the highest presence. Only models with significant correlations are displayed. The dotted lines and the sky-blue areas represent the regression lines and confidence intervals estimated using the PGLS models, respectively
Discussion
Our results confirmed that rockfishes with dominance of bright colour categories such as red, orange, and yellow, tend to inhabit deeper waters and have slower growth rates than their congeners with dominance of black, grey, and brown colour categories. Dark-coloured rockfishes are not found at depths deeper than 500 m, where only rockfishes with dominance of red categories are found (Fig. 5). The segregation of bright and dark-coloured rockfishes along depth hints at a photoprotective strategy to mitigate the potential down-regulation of immune responses induced by the UV irradiation in the shallow layers of water (Lawrence et al. 2020). This strategy is concordant with the contrasting photoprotective attributes of the chromatophores in fishes and their regulation under different levels of UV exposition (Mueller and Neuhauss 2014; Mizusawa et al. 2018). Melanophores, which produce dark colourations such as black, grey, and brown, can absorb UV radiations because of the melanin pigment, mitigating potential negative effects on rockfishes with dominance of dark-colour categories. In contrast, erythrophores and xanthophores, which contain carotenoids and coloured pteridines responsible for the bright colourations, do not exhibit the same photoprotective properties as melanophores. Carotenoids only have absorbances in the visible regions of the light spectra (400–520 nm) (blue–green) (Armstrong et al. 2000), while the photoprotective properties of coloured pteridines have not been conclusively proven yet. Hence, rockfishes with dominance of bright colours inhabit deeper waters to likely lessen their exposition to UV radiation in shallow waters. Besides melanophores, leucophores harbour colourless pteridine compounds with maximum absorbances at 320–330 nm (UV range) (Armstrong et al. 2000). These chromatophores are additional photoprotective components in species such as S. babcocki, S. cheni, S. owstoni, S. rubrivinctus, S. schlegelii, S. vulpes, and S. zonatus, in which pale-colour categories were found at percentages between 30 to 50% (Fig. 2).
Despite their variable depth distributions as adults, rockfishes often recruit in shallow environments with low-relief rocks, macrophytes, and seagrass beds (Love et al. 1991). However, recruitment in shallow environments comes with a trade-off for bright-coloured rockfishes because the long wavelengths of their body colours (red, orange, etc.) are still reflected (Marshall et al. 2019), making them more visible and susceptible to predation, while reducing their foraging success (Zhuang et al. 2020; Deville et al. 2023a). Conversely, in deeper environments, species with dominance of red- and orange-colour categories appear visually black due to the increasing light attenuation at deeper waters (Marshall et al. 2019), which does not require individuals to perform escape responses as often as in shallow waters. Since they spend more time in deeper environments with lower temperatures and oxygen, their metabolic and growth rates decrease (Cailliet et al. 2001; Black et al. 2021). The ecological consequences of rockfishes’ colourations align with expectations under environment-contingent natural selection, where colour polymorphism experiences varying degrees of fitness in response to different light environments (Gray and McKinnon 2007; Seehausen et al. 2008).
Brightly coloured rockfishes are endowed with morphological adaptations such as wider eyes, and narrower pectoral fins, which facilitate capturing more photons for the detection of prey and/ or predators in dim-light environments, and perform faster predatory and escape movements, respectively (Fulton et al. 2005; de Busserolles et al. 2020). Larger lower jaws come into play in species with high dominance of the first colour category (e.g. S. miniatus, S. phillipsi, etc.) (Tables 1 and 2, Figs. 2 and 7), allowing them to produce stronger bite forces than species with coloured irregular patches (Westneat 2003; de Busserolles et al. 2020). Meanwhile, the presence of irregular dark patches or spots in brightly coloured rockfishes (e.g. S. chlorostictus, S. elongatus, S. eos, S. rosenblatti, and S. umbrosus) (Fig. 2) can help them to obscure their shape, camouflaging them in the background and hampering the detection of their body contours in shallower environments (Cuthill et al. 2005; Stevens and Merilaita 2008). Other morphological adaptations in bright-coloured rockfishes include larger dorsal spines than in dark-coloured species, which allow the former to perform more effective defensive responses (Zhuang et al. 2020; Deville et al. 2023a). However, they were not assessed here due to the lack of data for most species.
Along the gradient of depth, the separation of bright and dark-coloured rockfishes in concordance with differences in growth rates and morphological traits (Table 2, Fig. 7), indicated that colouration differences are included in the correlational selection process across Sebastes rockfishes. This correlational selection has important implications for the diversification of rockfishes since combinations of certain colourations with specific values in life-history traits and morphological features may have led to changes in the genome structure and developmental pathways (Forsman et al. 2008; McKinnon and Pierotti 2010; Kolora et al. 2021). Bright-coloured rockfishes that dwell in deeper waters are endowed with other adaptations related to body colouration to live in their environments. Voronin et al. (2021) have pointed out that rockfishes in deeper waters have higher levels of specific lipids in their muscles that help them to maintain buoyancy while providing the necessary energy for performing vertical migrations through the water column. These lipid adaptations are possible through the differential expression of genes and pathways that control lipid metabolism and synthesis (Kolora et al. 2021). The presence of red- and orange-coloured rockfishes in the deeper ocean layers is related to these genetic variations because the metabolism, transport, and accumulation of carotenoids are tightly connected with the lipid metabolism (Yang et al. 2021). Moreover, the correlational selection includes positively selective genes responsible for DNA repair in bright-coloured rockfishes with large lifespans (e.g. S. aleutianus, S. aurora, and S. ruberrimus) (Heras et al. 2015; Kolora et al. 2021). These positively selected genes may come into play whenever these rockfishes undertake vertical migrations, mitigating the potential negative effects of UV radiation exposure in shallow environments.
Although genomic evidence regarding the development of colourations in rockfishes is not presented here, comparative studies in fishes displaying similar colourations anticipate that genes associated with carotenoid transport, deposition, and xanthophores formation may be upregulated in species with high dominance of red categories (e.g. S. aurora, S. mentella, S. polyspinis, S. variegatus, etc.) (Fig. 2), while genes responsible for melanin synthesis should be highly expressed in species with dominance of black categories (e.g. S. ijimae, S. ventricosus, S. vulpes, etc.) (Fang et al. 2022). Furthermore, the fact that juveniles of bright- and dark-coloured rockfishes feed on prey items containing carotenoids (Love et al. 1991) supports the idea that their differentiation in dominant colourations is due to the differential absorption of carotenoids from their shared prey, which can only occur through the differential expression of genes involved in carotenoid metabolism or xanthophores synthesis.
The analyses of species pairs confirmed that colour differences are initially correlated with dissimilarity in depth distributions. Most species pairs displayed only slight differences in their dominant colour categories (e.g. S. flammeus and S. iracundus, S. ijimae and S. zonatus, etc.) (Fig. 6), reflecting the moderate level of phylogenetic signal in the PCoA1. Only a pair of sister species (S. rubrivinctus and S. serriceps) displayed the divergence of reddish and blackish colours in the dominant categories (Fig. 2), which suggests that the main divergence in rockfishes with dominance of dark and bright colour categories in shallower and deeper species, respectively, took place over 3 million years ago (i.e.phylogenetic distance > 6) (Fig. 6). This divergence is coincident with the evolution of fundamental ecological morphotypes, including those associated with the differentiation between shallow reef and deep reef species (Gunderson and Vetter 2006; Hyde and Vetter 2007). Relatively recent divergences between rockfishes with dominance of dark and bright colourations have been reported when comparing ecotypes within two species inhabiting the northwestern Pacific (S. vulpes and S. zonatus) (Muto et al. 2019) and within the only two species reported in the southern hemisphere (S. capensis and S. oculatus) (Venerus et al. 2013). Of them, the divergence between dark and bright ecotypes within S. oculatus hypothetically occurred less than 350, 000 years ago, with the dark-coloured ecotype found in shallower environments than the bright-coloured ecotype (Venerus et al. 2013).
The analyses of species triads showed that colour and phylogenetic distances were not correlated. This finding disagrees with expectations where genetic stochastic mechanisms such as gene drift and mutation, lead to the establishment of defined colour differences between diverging populations in scenarios of sympatric speciation (Forsman et al. 2008). The random arrangement of dissimilarities in depth distribution and colour distances indicates, once again, that the asymmetric depth distributions of rockfishes are solely an initial condition for their speciation (Ingram 2011). Significant genetic divergences in fast-evolving markers suggest that colour distinctions between closely related species would function as prezygotic isolating barriers (Hyde and Vetter 2007). However, the lack of increase in colour differences from species pairs to triads (Fig. 3) also suggest that in the case of rockfishes, colouration may not be an important trait for identifying conspecifics at depth since there is limited available light. Hence, colour differences may only be effective prezygotic isolating barriers when combined with other traits like body size, and the utilisation of acoustic, chemical, and visual communication systems, and within specific scenarios of assortative mating (Schumer et al. 2017; Deville et al. 2023b). Moreover, considering the reports of hybridization within the genus (Valentin et al. 2002; Muto et al. 2013b; Buonaccorsi et al. 2005; Burford 2009; Saha et al. 2017; Keller et al. 2022; Deville et al. 2023b), the colour differences between closely related species are not increasing (Fig. 3) because of heterozygote advantage (Forsman et al. 2008) or because the divergence between closely related species is progressing independently of their colour differences (Stankowski and Ravinet 2021).
The exploration of colour differences estimated here was straightforward. However, it is important to acknowledge that the colour differences estimated here do not account for the spatial distribution of colour categories, particularly regarding the comparison of “homologous” pixels across species. To gain a more comprehensive understanding of these variations, it is essential to perform analyses of colour distribution, especially when dealing with closely related species that share similar dominant colour categories but differ in colouration patterns (Kronforst and Papa 2015; Weller and Westneat 2019). Taking for instance, the case of the species pair S. kiyomatsui and S. scythropus, which exhibit a similar reddish/pinkish dominant colour category, yet these species can be distinguished by unique coloured patches (Kai and Nakabo 2004) that had the highest values of presence after the most dominant colour category in each respective species (Fig. 2). However, in the context of this study, the quantification of colours using the colordistance package was chosen as the preferred approach over spatial methods like patternize (Van Belleghem et al. 2018) because of the remarkable diversity of body colours within Sebastes. By these means, it was ensured a more general exploration of colour variations and distinctions amongst species.
Conclusions
Our analyses confirmed the expectations that bright-coloured rockfishes inhabit deeper waters and have slower growth rates than dark-coloured rockfishes. The presence of bright-coloured rockfishes in deeper waters may represent a photoprotective strategy against the potential negative effects of UV radiation and the higher predation risk and predatory difficulty for these species in shallower environments. However, these rockfishes are endowed with morphological adaptations to deal with the challenging conditions in these environments. The separation of body colourations along with differences in growth rate and morphological traits confirmed that the correlational selection occurring in rockfishes includes body colouration. This selection is expected to lead to changes in genome structure and development pathways that include lipid and pigments metabolisms, and chromatophores development. The analyses of species pairs and triads indicated that depth is an initial driver of colour differentiation and that colour differences do not progressively increase with genetic divergences within triads. With these analyses, it was observed that the main divergence between bright- and dark-coloured rockfishes was related to the divergence of fundamental ecological morphotypes. Overall, this study emphasizes the intricate relationship between genetics, adaptation, metabolism, and the striking diversity of body colourations observed in rockfishes.
Data availability
The datasets generated and/or analysed during the current study can be downloaded from figshare using the https://doi.org/https://doi.org/10.6084/m9.figshare.24873342. Links for downloading photos used in this work are also available in the Supplementary Information Table S1.
References
Armstrong TN, Cronin TW, Bradley BP (2000) Microspectrophotometric analysis of intact chromatophores of the japanese medaka, Oryzias latipes. Pigment Cell Res 13:116–119. https://doi.org/10.1034/j.1600-0749.2000.130210.x
Artamonova VS, Makhrov AA, Karabanov DP, Rolskiy AYu, Bakay YuI, Popov VI (2013) Hybridization of beaked redfish (Sebastes mentella) with small redfish (Sebastes viviparus) and diversification of redfish (Actinopterygii: Scorpaeniformes) in the Irminger Sea. J Nat Hist 47:1791–1801. https://doi.org/10.1080/00222933.2012.752539
Black JA, Neuheimer AB, Horn PL, Tracey DM, Drazen JC (2021) Environmental, evolutionary, and ecological drivers of slow growth in deep-sea demersal teleosts. Mar Ecol Prog Ser 658:1–26. https://doi.org/10.3354/meps13591
Bouckaert RR, Drummond AJ (2017) bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol Biol 17:42. https://doi.org/10.1186/s12862-017-0890-6
Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, Heled J, Jones G, Kühnert D, Maio ND, Matschiner M, Mendes FK, Müller NF, Ogilvie HA, Plessis L du, Popinga A, Rambaut A, Rasmussen D, Siveroni I, Suchard MA, Wu C-H, Xie D, Zhang C, Stadler T, Drummond AJ (2019) BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLOS Computational Biology 15:e1006650. doi: https://doi.org/10.1371/journal.pcbi.1006650
Buonaccorsi VP, Kimbrell CA, Lynn EA, Vetter RD (2005) Limited realized dispersal and introgressive hybridization influence genetic structure and conservation strategies for brown rockfish, Sebastes auriculatus. Conserv Genet 6:697–713
Burford MO (2009) Demographic history, geographical distribution and reproductive isolation of distinct lineages of blue rockfish (Sebastes mystinus), a marine fish with a high dispersal potential. J Evol Biol 22:1471–1486. https://doi.org/10.1111/j.1420-9101.2009.01760.x
Cailliet GM, Andrews AH, Burton EJ, Watters DL, Kline DE, Ferry-Graham LA (2001) Age determination and validation studies of marine fishes: do deep-dwellers live longer? Exp Gerontol 36:739–764. https://doi.org/10.1016/S0531-5565(00)00239-4
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552. https://doi.org/10.1093/oxfordjournals.molbev.a026334
Cuthill IC, Stevens M, Sheppard J, Maddocks T, Párraga CA, Troscianko TS (2005) Disruptive coloration and background pattern matching. Nature 434:72–74. https://doi.org/10.1038/nature03312
de Busserolles F, Fogg L, Cortesi F, Marshall J (2020) The exceptional diversity of visual adaptations in deep-sea teleost fishes. Semin Cell Dev Biol 106:20–30. https://doi.org/10.1016/j.semcdb.2020.05.027
Deville D, Kawai K, Fujita H, Umino T (2023a) Ecomorphology of three closely related Sebastes rockfishes with sympatric occurrence in Seto Inland Sea, Japan. Hydrobiologia 850:1–18. https://doi.org/10.1007/s10750-023-05286-4
Deville D, Kawai K, Fujita H, Umino T (2023b) Genetic divergences and hybridization within the Sebastes inermis complex. PeerJ 11:e16391. https://doi.org/10.7717/peerj.16391
dos Anjos LEF, Gawryszewski FM, Bessa E (2022) Water column use by reef fishes of different color patterns. Neotrop Ichthyol 20:e210101. https://doi.org/10.1590/1982-0224-2021-0101
Fang W, Huang J, Li S, Lu J (2022) Identification of pigment genes (melanin, carotenoid and pteridine) associated with skin color variant in red tilapia using transcriptome analysis. Aquaculture 547:737429. https://doi.org/10.1016/j.aquaculture.2021.737429
Forsman A, Ahnesjö J, Caesar S, Karlsson M (2008) A model of ecological and evolutionary consequences of color polymorphism. Ecology 89:34–40. https://doi.org/10.1890/07-0572.1
Froese R (2022) Estimating somatic growth of fishes from maximum age or maturity. Acta Ichthyol Piscat 52:125–133. https://doi.org/10.3897/aiep.52.80093
Froese R, Pauly D (2023) FishBase. World Wide Web electronic publication. www.fishbase.org (06/2023)
Fulton CJ, Bellwood DR, Wainwright PC (2005) Wave energy and swimming performance shape coral reef fish assemblages. Proc R Soc B Biol Sci 272:827–832. https://doi.org/10.1098/rspb.2004.3029
Gamble S, Lindholm AK, Endler JA, Brooks R (2003) Environmental variation and the maintenance of polymorphism: the effect of ambient light spectrum on mating behaviour and sexual selection in guppies. Ecol Lett 6:463–472. https://doi.org/10.1046/j.1461-0248.2003.00449.x
Gray SM, McKinnon JS (2007) Linking color polymorphism maintenance and speciation. Trends Ecol Evol 22:71–79. https://doi.org/10.1016/j.tree.2006.10.005
Gunderson DR, Vetter RD (2006) Chapter 3. Temperate rocky reef fishes. In: Kritzer JP, Sale PF (eds) Marine metapopulations. Academic Press, Burlington, pp 69–117. https://doi.org/10.1016/B978-012088781-1/50006-6
Heras J, Aguilar A (2019) Comparative transcriptomics reveals patterns of adaptive evolution associated with depth and age within marine rockfishes (Sebastes). J Hered 110:340–350. https://doi.org/10.1093/jhered/esy070
Heras J, McClintock K, Sunagawa S, Aguilar A (2015) Gonadal transcriptomics elucidate patterns of adaptive evolution within marine rockfishes (Sebastes). BMC Genomics 16:656. https://doi.org/10.1186/s12864-015-1870-0
Hyde JR, Vetter RD (2007) The origin, evolution, and diversification of rockfishes of the genus Sebastes (Cuvier). Mol Phylogenet Evol 44:790–811. https://doi.org/10.1016/j.ympev.2006.12.026
Hyde JR, Kimbrell CA, Budrick JE, Lynn EA, Vetter RD (2008) Cryptic speciation in the vermilion rockfish (Sebastes miniatus) and the role of bathymetry in the speciation process. Mol Ecol 17:1122–1136. https://doi.org/10.1111/j.1365-294X.2007.03653.x
Ingram T (2011) Speciation along a depth gradient in a marine adaptive radiation. Proc R Soc B Biol Sci 278:613–618. https://doi.org/10.1098/rspb.2010.1127
Ingram T (2015) Diversification of body shape in Sebastes rockfishes of the north-east Pacific. Biol J Linn Soc 116:805–818. https://doi.org/10.1111/bij.12635
Ingram T, Kai Y (2014) The geography of morphological convergence in the radiations of Pacific Sebastes rockfishes. Am Nat 184:E115-131. https://doi.org/10.1086/678053
Ingram T, Shurin JB (2009) Trait-based assembly and phylogenetic structure in northeast Pacific rockfish assemblages. Ecology 90:2444–2453. https://doi.org/10.1890/08-1841.1
Kai Y, Nakabo T (2004) A new species of Sebastes (Scorpaeniformes: Scorpaenidae) from the Pacific coast of southern Japan. Ichthyol Res 51:5–9. https://doi.org/10.1007/s10228-003-0184-4
Kai Y, Nakabo T (2008) Taxonomic review of the Sebastes inermis species complex (Scorpaeniformes: Scorpaenidae). Ichthyol Res 55:238–259. https://doi.org/10.1007/s10228-007-0029-7
Kai Y, Nakabo T (2013) Taxonomic review of the Sebastes pachycephalus complex (Scorpaeniformes: Scorpaenidae). Zootaxa 3637:541–560
Kekäläinen J, Huuskonen H, Kiviniemi V, Taskinen J (2010) Visual conditions and habitat shape the coloration of the Eurasian perch (Perca fluviatilis L.): a trade-off between camouflage and communication? Biol J Lin Soc 99:47–59. https://doi.org/10.1111/j.1095-8312.2009.01339.x
Keller AA, Harms JH, Elz A, Wallace JR, Benante JA, Chappell A (2022) A tale of two species: Vermilion and sunset rockfish in the Southern California Bight. Fish Res 250:106275. https://doi.org/10.1016/j.fishres.2022.106275
Kolora SRR, Owens GL, Vazquez JM, Stubbs A, Chatla K, Jainese C, Seeto K, McCrea M, Sandel MW, Vianna JA, Maslenikov K, Bachtrog D, Orr JW, Love M, Sudmant PH (2021) Origins and evolution of extreme life span in Pacific Ocean rockfishes. Science 374:842–847. https://doi.org/10.1126/science.abg5332
Kronforst MR, Papa R (2015) The functional basis of wing patterning in Heliconius Butterflies: The molecules behind mimicry. Genetics 200:1–19. https://doi.org/10.1534/genetics.114.172387
Kumbar J, Ganesh CB (2021) Alpha-melanocyte stimulating hormone immunoreactivity in the brain of the cichlid fish Oreochromis mossambicus. Neuropeptides 87:102128. https://doi.org/10.1016/j.npep.2021.102128
Lande R, Arnold SJ (1983) The measurement of selection on correlated characters. Evolution 37:1210–1226. https://doi.org/10.2307/2408842
Lawrence KP, Young AR, Diffey BL, Norval M (2020) The impact of solar ultraviolet radiation on fish: Immunomodulation and photoprotective strategies. Fish Fish 21:104–119. https://doi.org/10.1111/faf.12420
Lehtonen TK, Candolin U, Wong BBM (2023) Environmental variation promotes colour morph-specific behavioural differences in a cichlid fish. Anim Behav 197:123–130. https://doi.org/10.1016/j.anbehav.2023.01.005
Love MS, Carr MH, Haldorson LJ (1991) The ecology of substrate-associated juveniles of the genus Sebastes. Environ Biol Fish 30:225–243. https://doi.org/10.1007/BF02296891
Love S, Lane S, Bowers B (2006) FileMaker 8 functions and scripts desk reference. Pearson Education, Ucrania, p 456
Marshall NJ, Cortesi F, de Busserolles F, Siebeck UE, Cheney KL (2019) Colours and colour vision in reef fishes: past, present and future research directions. J Fish Biol 95:5–38. https://doi.org/10.1111/jfb.13849
McKinnon JS, Pierotti MER (2010) Colour polymorphism and correlated characters: genetic mechanisms and evolution. Mol Ecol 19:5101–5125. https://doi.org/10.1111/j.1365-294X.2010.04846.x
McLean CA, Stuart-Fox D (2014) Geographic variation in animal colour polymorphisms and its role in speciation. Biol Rev 89:860–873. https://doi.org/10.1111/brv.12083
Mizusawa K, Kasagi S, Takahashi A (2018) Melanin-concentrating hormone is a major substance mediating light wavelength-dependent skin color change in larval zebrafish. Gen Comp Endocrinol 269:141–148. https://doi.org/10.1016/j.ygcen.2018.09.006
Mueller KP, Neuhauss SCF (2014) Sunscreen for fish: Co-option of UV light protection for camouflage. PLoS ONE 9:e87372. https://doi.org/10.1371/journal.pone.0087372
Muto N, Noda T, Kai Y, Nakabo T (2013a) First record of albinism in the rockfish Sebastes pachycephalus complex (Scorpaeniformes: Scorpaenidae). Ichthyol Res 60:195–197. https://doi.org/10.1007/s10228-012-0320-0
Muto N, Kai Y, Noda T, Nakabo T (2013b) Extensive hybridization and associated geographic trends between two rockfishes Sebastes vulpes and S. zonatus (Teleostei: Scorpaeniformes: Sebastidae). J Evol Biol 26:1750–1762. https://doi.org/10.1111/jeb.12175
Muto N, Takayama K, Kai Y (2016) First record of abnormal body coloration in a rockfish Sebastes trivittatus (Scorpaenoidei: Sebastidae). Ichthyol Res 63:197–199. https://doi.org/10.1007/s10228-015-0471-x
Muto N, Kai Y, Nakabo T (2019) Taxonomic review of the Sebastes vulpes complex (Scorpaenoidei: Sebastidae). Ichthyol Res 66:9–29. https://doi.org/10.1007/s10228-018-0641-8
Myoung SH, Myoung J-G, Jawad LA, Kim MJ, Park JM (2022) First report of abnormal body coloration in Sebastes koreanus (Actinopterygii: Perciformes: Sebastinae). Acta Ichthyol Piscat 52:209–213. https://doi.org/10.3897/aiep.52.89592
Narum SR, Buonaccorsi VP, Kimbrell CA, Vetter RD (2004) Genetic divergence between gopher rockfish (Sebastes carnatus) and black and yellow rockfish (Sebastes chrysomelas). Copeia 2004:926–931
Nishimoto MM, Washburn L, Love MS, Schroeder DM, Emery BM, Kui L (2019) Timing of juvenile fish settlement at offshore oil platforms coincides with water mass advection into the Santa Barbara Channel, California. Bull Mar Sci 95:559–582. https://doi.org/10.5343/bms.2018.0068
Orr JW, Brown MA, Baker DC (2000) Guide to rockfishes (Scorpaenidae) of the genera Sebastes, Sebastolobus, and Adelosebastes of the Northeast Pacific Ocean, 2nd edn. NOAA Tech. Memo. NMFS-AFSC-117, NMFS, Seattle, WA, p 47
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884. https://doi.org/10.1038/44766
Pandolfi M, Cánepa M, Ravaglia M, Maggese M, Paz D, Vissio P (2003) Melanin-concentrating hormone system in the brain and skin of the cichlid fish Cichlasoma dimerus: anatomical localization, ontogeny and distribution in comparison to α-melanocyte-stimulating hormone-expressing cells. Cell Tissue Res 311:61–69. https://doi.org/10.1007/s00441-002-0654-4
Paradis E, Schliep K (2019) ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35:526–528. https://doi.org/10.1093/bioinformatics/bty633
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Revell LJ (2024) phytools 2.0: an updated R ecosystem for phylogenetic comparative methods (and other things). PeerJ 12:e16505. https://doi.org/10.7717/peerj.16505
Roques S, Duchesne P, Bernatchez L (1999) Potential of microsatellites for individual assignment: the North Atlantic redfish (genus Sebastes) species complex as a case study. Mol Ecol 8:1703–1717. https://doi.org/10.1046/j.1365-294x.1999.00759.x
Saha A, Hauser L, Hedeholm R, Planque B, Fevolden S-E, Boje J, Johansen T (2017) Cryptic Sebastes norvegicus species in Greenland waters revealed by microsatellites. ICES J Mar Sci 74:2148–2158. https://doi.org/10.1093/icesjms/fsx039
Salis P, Lorin T, Laudet V, Frédérich B (2019) Magic traits in magic fish: understanding color pattern evolution using reef fish. Trends Genet 35:265–278. https://doi.org/10.1016/j.tig.2019.01.006
Schumer M, Powell DL, Delclós PJ, Squire M, Cui R, Andolfatto P, Rosenthal GG (2017) Assortative mating and persistent reproductive isolation in hybrids. Proc Natl Acad Sci 114:10936–10941. https://doi.org/10.1073/pnas.1711238114
Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HDJ, Miyagi R, van der Sluijs I, Schneider MV, Maan ME, Tachida H, Imai H, Okada N (2008) Speciation through sensory drive in cichlid fish. Nature 455:620–626. https://doi.org/10.1038/nature07285
Shum P, Pampoulie C, Sacchi C, Mariani S (2014) Divergence by depth in an oceanic fish. PeerJ 2:e525. https://doi.org/10.7717/peerj.525
Sidlauskas B (2008) Continuous and arrested morphological diversification in sister clades of characiform fishes: a phylomorphospace approach. Evolution 62:3135–3156. https://doi.org/10.1111/j.1558-5646.2008.00519.x
Sivasundar A, Palumbi SR (2010) Parallel amino acid replacements in the rhodopsins of the rockfishes (Sebastes spp.) associated with shifts in habitat depth. J Evol Biol 23:1159–1169. https://doi.org/10.1111/j.1420-9101.2010.01977.x
Stankowski S, Ravinet M (2021) Defining the speciation continuum. Evolution 75:1256–1273. https://doi.org/10.1111/evo.14215
Stefánsson MÖ, Reinert J, Sigurðsson Þ, Kristinsson K, Nedreaas K, Pampoulie C (2009) Depth as a potential driver of genetic structure of Sebastes mentella across the North Atlantic Ocean. ICES J Mar Sci 66:680–690. https://doi.org/10.1093/icesjms/fsp059
Stevens M, Merilaita S (2008) Defining disruptive coloration and distinguishing its functions. Philos Trans R Soc B Biol Sci 364:481–488. https://doi.org/10.1098/rstb.2008.0216
Treaster S, Deelen J, Daane JM, Murabito J, Karasik D, Harris MP (2023) Convergent genomics of longevity in rockfishes highlights the genetics of human life span variation. Sci Adv 9:eadd2743. https://doi.org/10.1126/sciadv.add2743
Tuset VM, Imondi R, Aguado G, Otero-Ferrer JL, Santschi L, Lombarte A, Love M (2015) Otolith patterns of rockfishes from the northeastern pacific. J Morphol 276:458–469. https://doi.org/10.1002/jmor.20353
Tuset VM, Otero-Ferrer JL, Gómez-Zurita J, Venerus LA, Stransky C, Imondi R, Orlov AM, Ye Z, Santschi L, Afanasiev PK, Zhuang L, Farré M, Love MS, Lombarte A (2016) Otolith shape lends support to the sensory drive hypothesis in rockfishes. J Evol Biol 29:2083–2097. https://doi.org/10.1111/jeb.12932
Valentin A, Sévigny J-M, Chanut J-P (2002) Geometric morphometrics reveals body shape differences between sympatric redfish Sebastes mentella, Sebastes fasdatus and their hybrids in the Gulf of St Lawrence. J Fish Biol 60:857–875. https://doi.org/10.1111/j.1095-8649.2002.tb02414.x
Van Belleghem SM, Papa R, Ortiz-Zuazaga H, Hendrickx F, Jiggins CD, Owen McMillan W, Counterman BA (2018) patternize: An R package for quantifying colour pattern variation. Methods Ecol Evol 9:390–398. https://doi.org/10.1111/2041-210X.12853
Venerus LA, Ciancio JE, Riva-Rossi C, Gilbert-Horvath EA, Gosztonyi AE, Garza JC (2013) Genetic structure and different color morphotypes suggest the occurrence and bathymetric segregation of two incipient species of Sebastes off Argentina. Naturwissenschaften 100:645–658. https://doi.org/10.1007/s00114-013-1061-2
Voronin VP, Nemova NN, Ruokolainen TR, Artemenkov DV, Rolskii AY, Orlov AM, Murzina SA (2021) Into the deep: new data on the lipid and fatty acid profile of redfish Sebastes mentella inhabiting different depths in the Irminger Sea. Biomolecules 11:704. https://doi.org/10.3390/biom11050704
Weller HI, Westneat MW (2019) Quantitative color profiling of digital images with earth mover’s distance using the R package color distance. PeerJ 7:e6398. https://doi.org/10.7717/peerj.6398
Westneat MW (2003) A biomechanical model for analysis of muscle force, power output and lower jaw motion in fishes. J Theor Biol 223:269–281. https://doi.org/10.1016/S0022-5193(03)00058-4
Wilson JR, Broitman BR, Caselle JE, Wendt DE (2008) Recruitment of coastal fishes and oceanographic variability in central California. Estuar Coast Shelf Sci 79:483–490. https://doi.org/10.1016/j.ecss.2008.05.001
Yamanome T, Chiba H, Takahashi A (2007) Melanocyte-stimulating hormone facilitates hypermelanosis on the non-eyed side of the barfin flounder, a pleuronectiform fish. Aquaculture 270:505–511. https://doi.org/10.1016/j.aquaculture.2007.05.037
Yang B-T, Wen B, Ji Y, Wang Q, Zhang H-R, Zhang Y, Gao J-Z, Chen Z-Z (2021) Comparative metabolomics analysis of pigmentary and structural coloration in discus fish (Symphysodon haraldi). J Proteomics 233:104085. https://doi.org/10.1016/j.jprot.2020.104085
Zhang R, Drummond A (2020) Improving the performance of Bayesian phylogenetic inference under relaxed clock models. BMC Evol Biol 20:54. https://doi.org/10.1186/s12862-020-01609-4
Zhuang L, Ye Z, Zhang C (2015) Application of otolith shape analysis to species separation in Sebastes spp. from the Bohai Sea and the Yellow Sea, northwest Pacific. Environ Biol Fish 98:547–558. https://doi.org/10.1007/s10641-014-0286-z
Zhuang L, Zhang C, Liu Q, Ye Z (2020) Age, growth, reproductive biology, color pattern, and ontogenetic divergence of two closely related rockfishes (Sebastes koreanus and S. nudus) off the coasts of the Shandong Peninsula, China. J Ocean Limnol 38:204–225. https://doi.org/10.1007/s00343-019-8303-x
Funding
Open Access funding provided by Hiroshima University.
Author information
Authors and Affiliations
Contributions
D. Deville retrieved DNA sequences and photos, performed statistical analyses, and wrote the manuscript. K. Kawai compiled photos and information about species, provided useful ideas on research design, and edited the manuscript. T. Umino was the supervisor, came up with useful ideas on research design, and edited the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
D. Deville, K. Kawai, and T. Umino declared that they have no conflict of interest.
Additional information
Responsible Editor: K. D. Clements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deville, D., Kawai, K. & Umino, T. Quantitative analysis of body colouration in Sebastes rockfishes. Mar Biol 171, 123 (2024). https://doi.org/10.1007/s00227-024-04436-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04436-z