Abstract
Fan mussel (Pinna nobilis) is one of the largest bivalve species in the Mediterranean Sea. The situation of the species is critical as it faces widespread mass mortality attributed to pathogens in various parts of the Mediterranean Sea. The Sea of Marmara (SoM) offers a unique environment for fan mussel populations, with some areas hosting alive populations. This study aims to explore and describe new P. nobilis populations in the SoM that are known to be not affected by mortality. An area of 28,200 m2 at 47 stations along the 105 km coastline in the southern part of the SoM was explored using underwater visual transects. A total of 544 alive fan mussels were recorded during the underwater surveys, ranging in total shell height from 11.8 to 31.4 cm. The mean density was estimated as 5.3 ind 100 m−2 although maxima of 18.8 ind 100 m−2 were recorded in some stations. These density hotspots were distributed from the shoreline to a 10 m depth range and 100 m distance from the shoreline in sandy and seagrass meadow habitats. The presence of juveniles provided evidence of successful recruitment. The distribution pattern and recorded mortalities were attributed to hydrodynamic factors and intense human activities. Potential environmental factors (low salinity and temperature) in the SoM may control or delay the possible spread of the lethal pathogens. Favorable conditions result in mussels’ resilience and survival mechanisms. The SoM offer a promising larval reservoir for the recolonization of affected areas, such as those found in the Aegean Sea, through larval exportation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fan mussel (Pinna nobilis Linnaeus, 1758), belongs to the family Pinnidae and is one of the largest bivalve species in the Mediterranean Sea (Vicente 1990). This species native to the Mediterranean Sea has a wide distribution from Spain to the Sea of Marmara (SoM) (Butler et al. 1993; Vázquez-Luis et al. 2017a). Although it grows quite fast during its first years of life (Kersting and García-March 2017), its growth slows down in older ages. Individuals can reach an impressive shell height of 120 cm and live up to 50 years (Zavodnik et al. 1991; Rouanet et al. 2015). Fan mussels exhibit a diverse distribution, thriving in various habitats such as seagrass meadows, sandy, gravelly, and rocky environments, as well as in rhodolith beds (Katsanevakis 2006; Basso et al. 2015; Kersting and García-March 2017; Karadurmuş and Sarı 2022a). Abundant along the coastal zone, fan mussels are found at depths of up to 60 m (Kersting and García-March 2017). They prefer areas with good environmental factors and gentle currents (Vázquez-Luis et al. 2017b; Prado et al. 2021). An essential ecological role of the fan mussel is its filtering capacity, with each individual capable of processing estimated 6 liters of seawater per hour (Trigos et al. 2014), effectively removing suspended particles and contributing to the clarification of the surrounding environment (Basso et al. 2015). Additionally, the fan mussel provides an attachment surface for sessile organisms, promoting biodiversity (Rabaoui et al. 2009). The diet mainly consists of detritus, phytoplankton, zooplankton, and pollen dust (Davenport et al. 2011; Alomar et al. 2015).
A destructive and geographically widespread mass mortality event in the Mediterranean basin has affected P. nobilis populations since 2016 (Vázquez-Luis et al. 2017a; Kersting et al. 2019; Katsanevakis et al. 2021). The first mortalities were caused by the protozoan pathogen Haplosporidium pinnae (Catanese et al. 2018; Vázquez-Luis et al. 2017a), with further studies revealing the impact of bacteria such as Mycobacterium sp. or Vibrio sp. on mortality (Catanese et al. 2018; Künili et al. 2021; Grau et al. 2022; Carella et al. 2023). The infection-associated deaths rapidly spread from west to east, starting in Spain and reaching the eastern Mediterranean Sea within a span of fewer than three years (Vázquez-Luis et al. 2017a; Kersting et al. 2019; Katsanevakis et al. 2021). As far as current knowledge goes, the infection extent has reached the Dardanelles Strait (Özalp and Kersting 2020; Künili et al. 2021), representing a significant geographic spread. Mortality rates have reached 80–100% across the Mediterranean Sea in most places, including Dardanelles Strait (Kersting et al. 2019; Özalp and Kersting 2020; Katsanevakis et al. 2021; Scarpa et al. 2021). While some populations in the Mediterranean Sea have managed to remain pathogen-free, they are situated in geographically isolated and environmentally specific regions (Cabanellas-Reboredo et al. 2019; Kersting et al. 2019; Katsanevakis et al. 2021). It is important to note that although extremely high salinity levels in these regions act as a deterrent to the spread and survival of the pathogen, they are not immune to anthropogenic pressure, including factors such as eutrophication and anoxia (Cabanellas-Reboredo et al. 2019; Giménez-Casalduero et al. 2020). Local-scale threats such as habitat loss, tourism, anchoring, illegal fishing, coastal constructions, and several factors like poor environmental conditions, eutrophication, and anoxia primarily contribute to deaths in certain areas (Basso et al. 2015; Giménez-Casalduero et al. 2020; Öndes et al. 2020a; Karadurmuş and Sarı 2022a). The World Union for Conservation of Nature (IUCN) has categorized the P. nobilis as Critically Endangered (Kersting et al. 2019) due to the severe mass mortality and significant reduction in stock sizes. As recommended by the IUCN, high-density regions should be protected, and containment measures include any impact that could cause incidental deaths (Kersting et al. 2019). Due to the high risk of extinction and the sharp decline in its global population, critical populations are drastically protected within various international and national directives (Kersting et al. 2019). Türkiye first provided protection to this species in 1997, and it remains on the list of forbidden species in the country (Karadurmuş and Sarı 2022a).
The existence of high-density alive P. nobilis populations in the SoM (Öndes et al. 2020a; Çınar et al. 2021a, b; Acarlı et al. 2022; Karadurmuş and Sarı 2022a) amidst mass mortalities elsewhere in the Mediterranean Sea, is of paramount importance for the natural recovery and survival of the species. These healthy populations serve as crucial larval exporting areas, offering a potential source of larvae for recolonization in mortality-affected sites, such as those in the Aegean Sea (Kersting et al. 2020; Papadakis et al. 2023). This study aims to describe new fan mussel populations that are not affected by widespread mass extinctions in the SoM and to examine their ecological aspects with a holistic approach (spatial and bathymetric distribution, abundance, mortality, population status). This study sheds light on the factors contributing to their resilience and survival of P. nobilis in the face of mass mortality events, providing valuable insights into potential conservation strategies for other affected areas.
Materials and methods
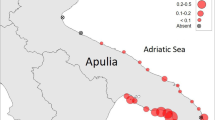
The study area covers Bandırma Bay and a large part of Kapıdağ Peninsula located in the south of the SoM (Fig. 1). In the study area, diving based on underwater observation was made at randomly determined stations at every 5 km intervals along the 105 km coastline. Additional dives were made in areas where critical density changes were observed. Underwater visual census includes techniques based on the assessment of habitat uses of target species and the determination of the abundance of target species within the transect area (Colvocoresses and Acosta 2007; Caldwell et al. 2016). These methods allow to calculate densities per unit area. Fan mussels were counted using the strip transect method in this study. This method gives more precise results since the boundaries are determined with precise strips and is frequently used in the estimation of fan mussel studies (García-March et al. 2002; Vafidis et al. 2014; Tsatiris et al. 2018; Karadurmuş and Sarı 2022a). Lead rope with a diameter of 3 mm and a length of 150 m, marked at intervals of five meters, was used as the transect material. The line was laid bottom vertically to the shore, starting from the shoreline. Two divers on either side of the strip used a 2 m wide lead made of plastic pipe with a center mark in their hands. The divers counted along the remaining strip in the transect area. In this way, two divers at each station scanned an area of 600 m2 (150 m × 4 m) in total. Divers recorded the number of individuals, the specimen status (dead or alive), depth, distance from shore, size of the individuals and the relative habitat type between the two marks by means of an underwater board for each segment.
During the census, the divers analyzed the living status of the individuals and recorded the alive and dead mussels counted in each subsection. Alive mussels respond by quickly closing their shell on approach or touch, while dead mussels contain no tissue and are unresponsive, their shells open and not closing. In addition, buried mussels with cracked or broken shells were defined as dead. Individuals completely plucked from sediment were not included in the count as they may have been carried indiscriminately by the flows. Recordings were taken throughout the entire field, including areas absent of fan mussels. The habitat type in each segment was classified and recorded as sandy, seagrass meadow, gravelly, rocky, shellfish, and muddy (IUCN 2023). Biometric measurements were made with a long-jaw multi-caliper and depth caliper with 0.1 cm division in randomly selected individuals along the transect. Measurements were made by taking the maximum width (W), minimum width (w), and maximum length (UL) measured from the unburied part of the shell. Based on these measurements; maximum shell length (Ht) was estimated (García-March et al. 2002). The Ht was calculated using the equation Ht = UL + h, and the regression (h) 1.79w + 0.5 was used for the buried shell length.
The mean fan mussel density at the stations was estimated as the individual number in per hundred square meters (ind 100 m−2). Density statuses were classified as low (< 5 ind 100 m−2), medium (5–10 ind 100 m−2), high (10–15 ind 100 m−2), and very high (> 15 ind 100 m−2) (Rabaoui et al. 2008; Karadurmuş and Sarı 2022a). All analyzes were performed separately for alive and dead individuals. The accumulated percentage of mortality (%) was calculated from the ratio of the number of dead individuals to the total number of individuals at each station. The variation of all density data according to depth, distance from the shore and habitat type was examined in the examined segments. Individuals with a shell length less than 20 cm were defined as juveniles, and those larger than 20 cm as adults (Richardson et al. 1999).
The data sets to be used in the study were examined in terms of suitability before the analysis. The assumptions of normality of variables and homogeneity of variances were evaluated with the Shapiro–Wilk test (Shapiro and Wilk 1965) and Levene’s test (Levene 1960). In the comparison of the quantitative data between two independent groups, parametric tests were used if the assumptions were significant, and non-parametric tests were used if the assumptions were not significant. The significance level for the test statistics of the hypotheses in both the assumption controls and parameter estimations was determined as α = 0.05 (Sokal and Rohlf 1969). Descriptive statistics (mean, standard error, quartiles, percentiles) were obtained according to the distribution structure for all data obtained. Data analysis and modeling was performed with SPSS v0.26.
Results
A total area of 28,200 m2 was scanned across 47 stations, revealing the presence of fan mussel individuals in only 17 of these stations. In total, 789 individuals were recorded, providing valuable insights into the distribution and abundance of fan mussels within the surveyed area. Among the recorded individuals, 544 (68.9%) were found to be alive, while 245 (31.1%) were observed in dead state. The mean alive mussel density was calculated as 5.3 ind 100 m−2 in the study area and five stations (S37, S44, S45, S46, and S47) were represented with above-average density. S45, located on the western side of the peninsula, exhibited the maximum mussel density with 18.8 ind 100 m−2. The total area covered by areas with fan mussels accounted for only 11.3% of the scanned area. At two specific stations (S37 and S45), very high-density clustering of fan mussels was recorded, while most of the remaining stations were classified as having low densities. Stations (S9, S12, S14, and S16) located in the eastern portion of the study area were represented by a very limited number of individuals (one or two individuals in each station) (Table 1).
This study revealed distinct patterns of spatial distribution and habitat preferences for P. nobilis. Distribution patterns indicate that the fan mussel can be found in various habitats along the scanned area, encompassing both shallow and deeper waters (down to 30.1 m). The 5–10 m depth contours had the highest alive density with 8.3 ind 100 m−2, followed by the 0–5 m depth contours with 6.1 ind 100 m−2. Dense clustering was recorded in populations reaching 9.9 ind 100 m−2 at a distance range of 50–75 m from the shore. Alive mussels most preferred sandy habitats with an average density of 13.6 ind 100 m−2, followed by seagrass habitats with an average density of 7.3 ind 100 m−2. The density of fan mussel was generally low in rocky, gravelly and shellfish habitats in this study. In contrast, P. nobilis was completely absent in muddy areas (Table 2).
The mean Ht was estimated as 20.1 ± 0.35 cm (11.8–31.4 cm) in alive and 23.2 ± 0.42 cm (11.8–32.5 cm) in dead individuals (Fig. 2). The ratio of juveniles to adults in the alive population was found to be evenly distributed (Fig. 3), with both comprising 50% of the population. A statistically significant difference in mean Ht was observed between alive and dead individuals (t(357) = − 5.737, p < 0.05). Accumulated mortality rates were recorded as 25.9% and 50.9% in juvenile and adult individuals, respectively.
Discussion
The study revealed the presence of thriving P. nobilis populations in the SoM, with evidence of successful recruitment and resilience to lethal pathogens. Despite the widespread mortality events in the Mediterranean Sea, the SoM continues to host alive individuals. Studies encompassing diverse regions, with a particular emphasis on the southern area of the SoM, revealed notable population densities: from 4.9 to 47.0 ind 100 m−2 in Acarlı et al. (2022), up to 71.2 ind 100 m−2 [alive] in Karadurmuş and Sarı (2022a), from 10 to 112 ind 100 m−2 [both alive and dead] in Acarlı et al. (2021a), from 6 to 240 ind 100 m−2 [alive] in Çınar et al. (2021a), and from 0.3 to 12 ind 100 m−2 in Çınar et al. (2021b), with a mean 24 ind 100 m−2 [alive] in Öndes et al. (2020b). Özalp and Kersting (2020) revealed the occurrence of mussel populations at high densities of up to 9 ind m−2 in shallow waters in the Dardanelles Strait, which connects the SoM with the Mediterranean Sea in the south. The patchy distribution pattern of the fan mussel in the study area implies localized concentrations rather than a homogenous distribution across the study area. The salinity difference between the Mediterranean Sea and the Black Sea causes water column stratification in the Dardanelles and Istanbul straits and the SoM. This stratification shapes the current system, with fresh Black Sea waters flowing on the surface towards the SoM and the Aegean Sea, while more saline Mediterranean Sea waters current towards the Black Sea at the bottom. The SoM exhibits lower temperature and salinity levels when contrasted with the Mediterranean Sea (Meriç et al. 2018). These environmental factors may play a role in regulating or impeding the potential spread of the pathogen, creating a natural sanctuary (Prado et al. 2021; Papadakis et al. 2023). Additionally, meteorological factors like rainfall, wind speed, and direction can influence the currents, potentially causing local deviations in the main current systems in the SoM (Beşiktepe et al. 1994; Meriç et al. 2018). The water current characteristic of the northern shores of the peninsula (from S18 to S36) and the eastern side of the bay (from S1 to S3) are clearly different from the other stations in the study area. This area is greatly affected by bottom currents from the Mediterranean and surface currents from the Black Sea (Fig. 1). So, the deprivation of populations at these stations can be associated with strong water currents. Because high currents in certain areas may create turbulent conditions and strong water flows that make it challenging for the mussels to maintain their attachment to the substrate. Hydrodynamic processes, including coastal currents, upwelling, and eddies, play a crucial role in the long-distance dispersal and connectivity between different populations of fan mussels (Kersting and García-March 2017; Kersting et al. 2020). These currents may also facilitate or prevent the dispersal and settlement of larvae (Kersting et al. 2020), thereby affecting the distribution of the species. Various researchers (Hendriks et al. 2011; Prado et al. 2021) have reported that fan mussel populations are vulnerable to severe weather events. As a defense mechanism, the mussels tend to avoid such areas and seek refuge in more sheltered habitats (Hendriks et al. 2011). The absence of individuals in areas with high currents is likely a result of the combined factors mentioned above.
The interior of Bandırma Bay is subjected to significant coastal uses and multiple industrial activities. The Bay is home to two heavy industry facilities, an international multi-purpose port with large berths, and a fishing port. The bay is also exposed to domestic pollution of the highly populated district through the deep discharge system (Özen et al. 2023). Heavy industry activities can introduce pollutants such as chemicals, heavy metals, and toxins into the water, which can have detrimental effects on the fan mussels’ health and survival. Most sites that still holding mussel populations are in highly anthropized areas, and a similar problem occurs at sites such as the Mar Menor in Spain (Giménez-Casalduero et al. 2020) and Gulf of Erdek in the SoM (Karadurmuş and Sarı 2022a). Additionally, domestic pollution can lead to eutrophication, oxygen depletion, and the accumulation of pollutants in the water, making it unsuitable for the fan mussels (Basso et al. 2015; Giménez-Casalduero et al. 2020). In addition, secondary bottom current and local surface current, which have a cyclical effect in the inner parts of the bay, prevent the pollution from leaving the bay and contribute to the continuity of the pollution pressure (Fig. 1). The bay also hosts the high-speed ferry route and busy anchorage area. Marine operations often involve negative impacts, which generate significant disturbances in the water and along the seabed (Öndes et al. 2020a). The absence or limited number of fan mussels at stations in this region, from S4 to S8, and in the bay is thought to be a result of the above-mentioned multi-faceted intense activities.
The western portion of the study area, spanning from S37 to S45, along with Fener Island (S46) and Hali Ada (S47) on the eastern side, both of which are isolated from the peninsula, exhibited fan mussel populations characterized by exceptionally high densities. Contrary to Acarlı et al. (2022) new discoveries of one or two individuals were obtained at the stations scanned in the area between S9 and S18 in Bandırma Bay. Previous research (Acarlı et al. 2022; Karadurmuş and Sarı 2022a) has also documented the presence of thriving populations in Erdek Bay, situated to the west of the peninsula, which further corroborates the region’s ability to support survival and settlement populations of live fan mussels. These areas boast more stable environmental conditions and exhibit lower water velocities, factors that are conducive to the successful survival and settlement of fan mussels. We hypothesize that salinity and temperature are potential factors behind the survival of populations in the SoM, as opposed to the Mediterranean Sea. The salinity of the SoM varies between 17.8 and 38.5 psu throughout the year and is characterized by an annual average of 22.5 psu. Cabanellas-Reboredo et al. (2019) reported that the onset of pathogen has been associated with temperatures above 13.5 °C and a narrow salinity range (36.5–39.7 psu). The fact that the SoM has a relatively lower temperature and salinity compared to the Mediterranean Sea may control or delay the possible spread of the pathogen. Notably, these regions remain entirely free from anthropogenic influences, preserving a sheltered and protected environment that further supports the proliferation of fan mussels. Understanding the complex relationships between environmental features and the distribution of fan mussels is crucial for effective conservation and management strategies. Further research in the study area should focus on understanding the multiple factors (environmental, physical, chemical, hydrodynamic, anthropogenic) that drive distribution patterns and explore additional potential areas that may contribute to the conservation of this species.
The distribution pattern of populations in the study area was mainly connected to depth and habitat type. Individuals were mostly concentrated in the shallow zone between the shoreline and 10 m, and the density decreased as depth increased. Similarly, areas of sandy bottom and seagrass meadow were also represented by a significant density of alive mussels. Habitats with high P. nobilis concentrations are very diverse and can be found in almost all habitat types down to depths of 40–50 m (Kersting and García-March 2017). Normally, seagrass areas are the primary habitat for fan mussel (Hendriks et al. 2011; Vázquez-Luis et al. 2014; Karadurmuş and Sarı 2022a). However, light transmittance decreases due to the high suspended solid load in the SoM, and, therefore, seagrass meadows can spread up to a maximum depth of 6–7 m (Cirik et al. 2006). Therefore, sandy habitats are the primary habitat for P. nobilis in the study area. Such depth (Šiletić and Peharda 2003; García-March et al. 2007a; Karadurmuş and Sarı 2022a) and habitat (Katsanevakis and Thessalou-Legaki 2009; Hendriks et al. 2011; Deudero et al. 2015; Acarlı et al. 2022; Karadurmuş and Sarı 2022a) related diversities have an important impact on fan mussels’ survival and recruitment. Key environmental factors such as hydrodynamics, light intensity, temperature, and productivity in shallow waters strongly support the existence of the species (Prado et al. 2014; Russo 2017; Tsatiris et al. 2018). Results indicating that sandy grounds and seagrass beds are the primary habitats where the species thrives. Sandy bottoms provide the most convenient environment for the implantation of byssus filaments (García-March et al. 2007b), while seagrass meadows ensure shelter against hydrodynamic factors (García-March and Kersting 2006; Hendriks et al. 2011). These habitats offer protection, food resources, and suitable substrate for attachment, making them important habitats for the species (Vázquez-Luis et al. 2014). Muddy substrates seem to be unsuitable for attachment and may not provide the necessary conditions for the survival and growth of the species (Katsanevakis 2006; Tsatiris et al. 2018).
Most of the current P. nobilis population were characterized by a predominance of juveniles across all areas studied. This situation often implies successful recruitment and reproduction and can generally indicate a stable population, good environmental conditions, and the absence of predation (Kersting and García-March 2017; Vázquez-Luis et al. 2017b). Kersting and García-March (2017) reported a scarcity of individuals below 45 cm in Ht in the population of the Columbretes Islands (NW Mediterranean). They emphasized that such a distribution model indicates that it can increase the survival rate and that this size may be a refuge size. On the other hand, the recorded mortalities are alarming, and as stated in previous studies (Öndes et al. 2020b; Çınar et al. 2021a, b; Acarlı et al. 2021a, 2022; Karadurmuş and Sarı 2022a), mortalities in P. nobilis have been recorded in the SoM due to multiple factors such as fisheries, anchoring, tourism, and diving activities. Although it is well known that environmental factors and human effects cause mortalities, it is still unknown whether pathogens, parasites, or other disease-causing agents are responsible for deaths in the SoM (Karadurmuş and Sarı 2022a; Papadakis et al. 2023). The size distribution revealed that larger size older individuals were represented in fewer numbers in the population (Fig. 3). Adults in the large size group were more affected by mortalities, thus leading to slow population dynamics and to the species’ vulnerability to unexpected events. Juveniles may indicate inclusion in the productive stock, and rapid growth in the first years of life may help shorten the period of vulnerability of the stock (Kersting and García-March 2017). Juveniles may exhibit a high survival rate due to their superior responsiveness to pathogens (Šarić et al. 2020). The presence of pathogens that cause mass deaths in the Mediterranean is not yet known in the study area. As far as we know best, the last spread of the disease is limited to the Dardanelles Strait (Özalp and Kersting 2020; Künili et al. 2021). In the most recent occurrence, a significant marine mucilage outbreak took place in the SoM, spanning from November 2020 to August 2021. Consequently, numerous benthic species within marine communities experienced extensive mass mortality (Karadurmuş and Sarı 2022b; Karakulak et al. 2023). This phenomenon proved fatal to pelagic fish and crustaceans, with individuals being severely affected by suffocation due to the presence of mucilage or anoxia (Karadurmuş and Sarı 2022b). This devastating event may cover the benthic habitats where bivalves like P. nobilis reside, potentially smothering them and reducing their access to food and oxygen. The phenomena can alter the availability and distribution of planktonic food sources for bivalves, which might affect their feeding and distribution patterns. Acarlı et al. (2021b) claimed that the mucilage event in the SoM may have a negative impact on fan mussel populations either directly or through habitat loss (especially seagrass meadows). There is no conclusive evidence that mucilage is responsible for fan mussel deaths, although various potential effects have been predicted.
Conclusion
This study demonstrates the discovery of new P. nobilis populations in addition to previous studies in the south of the SoM. Findings present a unique opportunity to investigate the factors contributing to the mussel’s resilience and survival capacity in the face of widespread extinction events. Despite the concerning situation observed in many areas, the SoM continues to serve as a vital refuge for fan mussel populations, with certain regions supporting thriving and high-density communities. The SoM can play a vital role in facilitating natural recoveries in sites affected by mortality events through larval export (Kersting et al. 2020; Papadakis et al. 2023). The presence of juveniles indicates successful recruitment and settlement, but the higher mortality rates among adults raise significant concerns. The fact that the SoM is characterized by lower temperature and salinity than the Mediterranean appears to be the main factors preventing the entry of the pathogen, but further research is still needed. The lessons learned from the survival of these populations could provide valuable insights into guide conservation strategies aimed at rejuvenating the species in its former Mediterranean Sea habitats. To ensure the conservation and survival of P. nobilis, continued monitoring and in-depth studies on the species are imperative, including investigations into potential threats from pathogens and anthropogenic activities. While this study sheds light on the southern part of the SoM, it is crucial to conduct additional research covering the entire of the SoM to gain a comprehensive understanding of fan mussel populations in the region. Immediate investigation into the presence of pathogens causing mortality is strongly recommended to address potential threats to the species’ survival. By undertaking such efforts, we can enhance our ability to safeguard this ecologically significant species and contribute to the preservation of biodiversity in the Mediterranean Sea.
Data availability
Data will be made available on request.
References
Acarlı S, Acarlı D, Kale S (2021a) Current status of critically endangered fan mussel Pinna nobilis (Linnaeus 1758) population in Çanakkale Strait, Turkey. Mar Sci Tech Bull 10:62–70. https://doi.org/10.33714/masteb.793885
Acarlı S, Acarlı D, Kale S (2021b) The effects of mucilage event on the population of critically endangered Pinna nobilis (Linnaeus 1758) in Ocaklar Bay (Marmara Sea, Turkey). Acta Nat Sci 2:148–158. https://doi.org/10.29329/actanatsci.2021.350.09
Acarlı D, Acarlı S, Kale S (2022) The struggle for life: Pinna nobilis in the Marmara Sea (Turkey). Thalassas 38:1199–1212. https://doi.org/10.1007/s41208-022-00470-0
Alomar C, Vázquez-Luis M, Magraner K, Lozano L, Deudero S (2015) Evaluating stable isotopic signals at bivalve Pinna nobilis under different human pressures. J Exp Mar Biol Ecol 467:77–86. https://doi.org/10.1016/j.jembe.2015.03.006
Basso L, Vázquez-Luis M, García-March JR, Deudero S, Alvarez E et al (2015) The pen shell, Pinna nobilis: a review of population status and recommended research priorities in the Mediterranean Sea. Adv Mar Biol 71:109–160. https://doi.org/10.1016/bs.amb.2015.06.002
Beşiktepe ŞT, Sur HI, Özsoy E, Latif MA, Oğuz T et al (1994) The circulation and hydrography of the Marmara Sea. Prog Oceanogr 34:285–334. https://doi.org/10.1016/0079-6611(94)90018-3
Butler A, Vicente N, Gaulejac B (1993) Ecology of the pterioid bivalves Pinna bicolor Gmelin and Pinna nobilis L. Mar Life 3:37–45
Cabanellas-Reboredo M, Vázquez-Luis M, Mourre B, Álvarez E, Deudero S et al (2019) Tracking the mass mortality outbreak of pen shell Pinna nobilis populations: a collaborative effort of scientists and citizens. Sci Rep 9:13355. https://doi.org/10.1038/s41598-019-49808-4
Caldwell ZR, Zgliczynski BJ, Williams GJ, Sandin SA (2016) Reef fish survey techniques: assessing the potential for standardizing methodologies. PLoS ONE 11:e0153066. https://doi.org/10.1371/journal.pone.0153066
Carella F, Palić D, Šarić T, Župan I, Gorgoglione B et al (2023) Multipathogen infections and multifactorial pathogenesis involved in noble pen shell (Pinna nobilis) mass mortality events: background and current pathologic approaches. Vet Pathol 60:560–577. https://doi.org/10.1177/03009858231186737
Catanese G, Grau A, Valencia JM, García-March JR, Vázquez-Luis M et al (2018) Haplosporidium pinnae sp. nov., a haplosporidian parasite associated with mass mortalities of the fan mussel, Pinna nobilis in the Western Mediterranean Sea. J Invertebr Pathol 157:9–24. https://doi.org/10.1016/j.jip.2018.07.006
Cirik Ş, Akçalı B, Özalp HB (2006) Determination of Posidonia oceanica borders at Dardanelle Strait and Marmara Sea by using marking method. EU J Fish Aquat Sci 23:45–48
Çınar M, Bilecenoğlu M, Yokeş M, Güçlüsoy H (2021a) Pinna nobilis in the south Marmara Islands (Sea of Marmara); it still remains uninfected by the epidemic and acts as egg laying substratum for an alien invader. Medit Mar Sci 22:161–168. https://doi.org/10.12681/mms.25289
Çınar M, Bilecenoğlu M, Yokeş M, Güçlüsoy H (2021b) The last fortress fell: mass mortality of Pinna nobilis in the Sea of Marmara. Medit Mar Sci 22:669–676. https://doi.org/10.12681/mms.27137
Colvocoresses J, Acosta A (2007) A large-scale field comparison of strip transect and stationary point count methods for conducting length-based underwater visual surveys of reef fish populations. Fish Res 85:130–141. https://doi.org/10.1016/j.fishres.2007.01.012
Davenport J, Ezgeta-Balic D, Peharda M, Skejic S, Nincevic-Gladan Z et al (2011) Size-differential feeding in Pinna nobilis L. (Mollusca: Bivalvia): exploitation of detritus, phytoplankton and zooplankton. Estuar Coast Shelf Sci 92:246–254. https://doi.org/10.1016/j.ecss.2010.12.033
Deudero S, Vázquez-Luis M, Álvarez E (2015) Human stressors are driving coastal benthic long-lived sessile fan mussel Pinna nobilis population structure more than environmental stressors. PLoS ONE 10:e0134530. https://doi.org/10.1371/journal.pone.0134530
García-March JR, Kersting DK (2006) Preliminary data on the distribution and density of Pinna nobilis and Pinna rudis in the Columbretes Islands Marine Reserve (Western Mediterranean, Spain). Org Divers Evol 6:6–16
García-March JR, García-Carrascosa AM, Luís Peña Á (2002) In situ measurement of Pinna nobilis shells for age and growth studies: a new device. Mar Ecol 23:207–217. https://doi.org/10.1046/j.1439-0485.2002.02781.x
García-March JR, García-Carrascosa AM, Peña Cantero AL, Wang YG (2007a) Population structure, mortality and growth of Pinna nobilis Linnaeus, 1758 (Mollusca, Bivalvia) at different depths in Moraira bay (Alicante, Western Mediterranean). Mar Biol 150:861–871. https://doi.org/10.1007/s00227-006-0386-1
García-March JR, Pérez-Rojas L, García-Carrascosa AM (2007b) Influence of hydrodynamic forces on population structure of Pinna nobilis L., 1758 (Mollusca: Bivalvia): the critical combination of drag force, water depth, shell size and orientation. J Exp Mar Biol Ecol 342:202–212. https://doi.org/10.1016/j.jembe.2006.09.007
Giménez-Casalduero F, Gomariz-Castillo F, Alonso-Sarría F, Cortés E, Izquierdo-Muñoz A et al (2020) Pinna nobilis in the Mar Menor coastal lagoon: a story of colonization and uncertainty. Mar Ecol Prog Ser 652:77–94. https://doi.org/10.3354/meps13468
Grau A, Villalba A, Navas JI, Hansjosten Z, Valencia JM et al (2022) Wide-geographic and long-term analysis of the role of pathogens in the decline of Pinna nobilis to critically endangered species. Front Mar Sci 9:666640. https://doi.org/10.3389/fmars.2022.666640
Hendriks IE, Cabanellas-Reboredo M, Bouma TJ, Deudero S, Duarte CM (2011) Seagrass meadows modify drag forces on the shell of the fan mussel Pinna nobilis. Estuar Coasts 34:60–67. https://doi.org/10.1007/s12237-010-9309-y
IUCN (2023) International Union for Conservation of Nature: Habitats Classification Scheme (Version 3.1). https://www.iucnredlist.org/resources/habitat-classification-scheme. Accessed 20 Aug 2023
Karadurmuş U, Sarı M (2022a) The last hope: the struggle for survival of fan mussels in the Gulf of Erdek, Sea of Marmara, Turkey. Medit Mar Sci 23:473–483. https://doi.org/10.12681/mms.28474
Karadurmuş U, Sarı M (2022b) Marine mucilage in the Sea of Marmara and its effects on the marine ecosystem: mass deaths. Turk J Zool 46:93–102. https://doi.org/10.3906/zoo-2108-14
Karakulak FS, Kahraman AE, Uzer U, Gül B, Doğu S (2023) Effects of mucilage on the fisheries in the Sea of Marmara. In: Albay M (ed) Mucilage problem in the Sea of Marmara. Istanbul University Press, Istanbul, pp 193–215
Katsanevakis S (2006) Population ecology of the endangered fan mussel Pinna nobilis in a marine lake. Endanger Species Res 1:1–9. https://doi.org/10.3354/esr001051
Katsanevakis S, Thessalou-Legaki M (2009) Spatial distribution, abundance and habitat use of the protected fan mussel Pinna nobilis in Souda Bay, Crete. Aquat Biol 8:45–54. https://doi.org/10.3354/ab00204
Katsanevakis S, Carella F, Çinar ME, Čižmek H, Jimenez C et al (2021) The fan mussel Pinna nobilis on the brink of extinction in the Mediterranean. Imperiled: The encyclopedia of conservation, reference module in earth systems and environmental sciences. Elsevier, Amsterdam, pp 700–709. https://doi.org/10.1016/B978-0-12-821139-7.00070-2
Kersting DK, García-March JR (2017) Long-term assessment of recruitment, early stages and population dynamics of the endangered Mediterranean fan mussel Pinna nobilis in the Columbretes Islands (NW Mediterranean). Mar Environ Res 130:282–292. https://doi.org/10.1016/j.marenvres.2017.08.007
Kersting D, Benabdi M, Čižmek H, Grau A, Jimenez C et al (2019) Pinna nobilis. The IUCN Red List of Threatened Species 2019:e.T160075998A160081499. https://doi.org/10.2305/IUCN.UK.2019-3.RLTS.T160075998A160081499.en. Accessed 5 Oct 2021
Kersting DK, Vázquez-Luis M, Mourre B, Belkhamssa FZ, Álvarez E et al (2020) Recruitment disruption and the role of unaffected populations for potential recovery after the Pinna nobilis mass mortality event. Front Mar Sci 7:594378. https://doi.org/10.3389/fmars.2020.594378
Künili İE, Gürkan SE, Aksu A, Turgay E, Çakır F et al (2021) Mass mortality in endangered fan mussels Pinna nobilis (Linnaeus 1758) caused by co-infection of Haplosporidium pinnae and multiple Vibrio infection in Çanakkale Strait, Turkey. Biomarkers 26:450–461. https://doi.org/10.1080/1354750x.2021.1910344
Levene H (1960) Contributions to probability and statistics: Essays in Honor of Harold Hotelling. Stanford University Press, Redwood City, CA
Meriç E, Yokeş MB, Yümün ZÜ, Eryılmaz M, Yücesoy-Eryılmaz F (2018) Alien benthic foraminifers from Turkish Strait System. Int J Environ Geoinform 5:68–75. https://doi.org/10.30897/ijegeo
Öndes F, Kaiser MJ, Güçlüsoy H (2020a) Human impacts on the endangered fan mussel, Pinna nobilis. Aquat Conserv Mar Freshw 30:31–41. https://doi.org/10.1002/aqc.3237
Öndes F, Alan V, Akçalı B, Güçlüsoy H (2020b) Mass mortality of the fan mussel, Pinna nobilis in Turkey (eastern Mediterranean). Mar Ecol 41:e12607. https://doi.org/10.1111/maec.12607
Özalp HB, Kersting DK (2020) A pan-Mediterranean extinction? Pinna nobilis mass mortality has reached the Turkish straits system. Mar Biodivers 50:81. https://doi.org/10.1007/s12526-020-01110-7
Özen Ç, Sarı E, Arslan Kaya TN, Gül M, Kurt MA et al (2023) Paleo environmental pollution assessment of Erdek and Bandırma Bays in the Sea of Marmara, Türkiye. Soil Sediment Contam 33(6):1–23. https://doi.org/10.1080/15320383.2023.2200863
Papadakis O, Mamoutos I, Ramfos A, Catanese G, Papadimitriou E et al (2023) Status, distribution, and threats of the last surviving fan mussel populations in Greece. Medit Mar Sci 24:679–708. https://doi.org/10.12681/mms.35384
Prado P, Caiola N, Ibáñez C (2014) Habitat use by a large population of Pinna nobilis in shallow waters. Sci Mar 78:555–565. https://doi.org/10.3989/scimar.04087.03A
Prado P, Grau A, Catanese G, Cabanes P, Carella F et al (2021) Pinna nobilis in suboptimal environments are more tolerant to disease but more vulnerable to severe weather phenomena. Mar Environ Res 163:105220. https://doi.org/10.1016/j.marenvres.2020.105220
Rabaoui L, Tlig-Zouari S, Hassine OKB (2008) Distribution and habitat of the fan mussel Pinna nobilis Linnaeus, 1758 (Mollusca: Bivalvia) along the northern and eastern Tunisian coasts. Cah Biol Mar 49:67–78.
Rabaoui L, Tlig-Zouari S, Cosentino A, Ben Hassine OK (2009) Associated fauna of the fan shell Pinna nobilis (Mollusca: Bivalvia) in the northern and eastern Tunisian coasts. Sci Mar 73:129–141. https://doi.org/10.3989/scimar.2009.73n1129
Richardson C, Kennedy H, Duarte C, Kennedy DP, Proud SV (1999) Age and growth of the fan mussel Pinna nobilis from south-east Spanish Mediterranean seagrass (Posidonia oceanica) meadows. Mar Biol 133:205–212. https://doi.org/10.1007/s002270050459
Rouanet E, Trigos S, Vicente N (2015) From youth to death of old age: the 50-year story of a Pinna nobilis fan mussel population at Port-Cros Island (Port-Cros National Park, Provence, Mediterranean Sea). Sci Rep Port Cros Natl Park 29:209–222
Russo P (2017) Lagoon malacofauna: results of malacological research in the Venice Lagoon. Boll Malacol 53:49–62
Šarić T, Župan I, Aceto S, Villari G, Palić D et al (2020) Epidemiology of noble pen shell (Pinna nobilis L. 1758) mass mortality events in Adriatic Sea is characterised with rapid spreading and acute disease progression. Pathogens 9:776. https://doi.org/10.3390/pathogens9100776
Scarpa F, Sanna D, Azzena I, Cossu P, Casu M (2021) From dark to light and back again: is Pinna nobilis, the largest Mediterranean shellfish, on the brink of extinction?. Veterinaria 70:1–14. https://doi.org/10.51607/22331360.2021.70.1.1
Shapiro SS, Wilk MB (1965) An analysis of variance test for normality (complete samples). Biometrika 52:591–611. https://doi.org/10.2307/2333709
Šiletić T, Peharda M (2003) Population study of the fan shell Pinna nobilis L. in Malo and Veliko Jezero of the Mljet National Park (Adriatic Sea). Sci Mar 67:91–98. https://doi.org/10.3989/scimar.2003.67n191
Sokal RR, Rohlf FJ (1969) Introduction to biostatistics. W.H. Freeman and Company, New York
Trigos S, Garcia-March JR, Vicente N, Tena J, Torres J (2014) Utilization of muddy detritus as organic matter source by the fan mussel Pinna nobilis. Medit Mar Sci 15:667–674. https://doi.org/10.12681/mms.836
Tsatiris A, Papadopoulos V, Makri D, Topouzelis K, Manoutsoglou E et al (2018) Spatial distribution, abundance and habitat use of the endemic Mediterranean fan mussel Pinna nobilis in Gera Gulf, Lesvos (Greece): comparison of design-based and model-based approaches. Medit Mar Sci 19:642–655. https://doi.org/10.12681/mms.14156
Vafidis D, Antoniadou C, Voultsiadou E, Chintiroglou C (2014) Population structure of the protected fan mussel Pinna nobilis in the south Aegean Sea (Eastern Mediterranean). J Mar Biol Assoc 94:787–796. https://doi.org/10.1017/S0025315413001902
Vázquez-Luis M, March D, Alvarez E, Alvarez-Berastegui D, Deudero S (2014) Spatial distribution modelling of the endangered bivalve Pinna nobilis in a marine protected area. Medit Mar Sci 15:626–634. https://doi.org/10.12681/mms.796
Vázquez-Luis M, Álvarez E, Barrajón A, García-March JR, Grau A et al (2017a) S.O.S. Pinna nobilis: a mass mortality event in western Mediterranean Sea. Front Mar Sci 4:220. https://doi.org/10.3389/fmars.2017.00220
Vázquez-Luis M, Álvarez E, Deudero S (2017b) Proposal of action plan for Pinna nobilis in the Mediterranean Sea in the frame of the Marine Strategy Framework Directive (MSFD). Instituto Español de Oceanografía, Centro Oceanográfico de Baleares, 53 p
Vicente N (1990) Estudio ecológico y protección del molusco lamelibranquio Pinna nobilis L., 1758 en la costa mediterránea. Iberus 9:269–279
Zavodnik D, Hrs-Brenko M, Legac M (1991) Synopsis on the fan shell Pinna nobilis L. in the eastern Adriatic Sea. In: Boudouresque CF, Avon M, Gravez V (eds) Les Espèces Marines à Protèger en Mèditerranèe. GIS Posidonie publications, Marseille, pp 169–178
Acknowledgements
The authors would like to thank first-class divers Tacan Benli, Rafet Bıçaklı, Gökhan Erdoğan and Samet Avcı who voluntarily accompanied the dives throughout the fieldwork.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This work was supported by Sustainable Benefit Program funded by Borusan Holding and Impact Hub Istanbul as part of the HOPE PINNA project.
Author information
Authors and Affiliations
Contributions
U.K.: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Original Draft, Visualization, Project administration. M.S.: Conceptualization, Methodology, Investigation, Writing—Review and Editing, Project administration, Funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This is an observational study. No ethical approval is required.
Additional information
Responsible Editor: S. Shumway.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karadurmuş, U., Benli, T. & Sarı, M. Discovering new living Pinna nobilis populations in the Sea of Marmara. Mar Biol 171, 90 (2024). https://doi.org/10.1007/s00227-024-04416-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04416-3