Abstract
Artificial reefs are used as tools for the restoration of degrading coral reefs by providing new settlement substrates. The initial recruitment process consists of the formation of microbial biofilms shortly after deployment. The aim of the present study is to compare biofilm composition and development on artificial substrates at two different coral-reef sites. These locations differ in their environmental conditions, including their level of anthropogenic impact. Substrate and seawater samples were collected four times during the first 6 months after deployment, using a new methodology termed ‘tab-by-tab’. DNA was extracted, sequenced, and sorted for both prokaryotic (16s) and eukaryotic (18s) genes. No difference was found between the planktonic communities in the water, yet significant differences were observed in the biofilm communities on the structures at the two sites. Moreover, differences were recorded in coral recruitment rates, which are known to be affected by biofilm composition. Our findings suggest a potential role of environmental conditions in the early biofilm stages (first few months), which in turn may impact the succession and development of coral-reef communities and the success of artificial reefs as restoration tools.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coral reefs provide vital resources to nearly half a billion people around the world (Cinner 2014), and have the highest economic value (per area unit) among all ecosystems (Costanza et al. 2014). However, the important ecosystem services provided by coral reefs are in decline, as they have been degrading globally over the last few decades (Burke et al. 2011; Hughes et al. 2017). At the current rate, experts predict that 86% of the world’s coral reefs will be severely and irreversibly damaged by 2040 (Heron et al. 2017).
Since conservation efforts alone have been found to be insufficient in reversing the decline (Deáth et al. 2012; Knowlton 2012), there is a growing need for active restoration (Rinkevich 2005; Abelson et al. 2016; Anthony et al. 2017). One commonly used restoration method is the deployment of artificial reefs (Clark and Edwards 1999; Pickering et al. 1999). Although this method has received some criticism over the years (Bohnsack 1989; Pickering and Whitmarsh 1997; Baine 2001; Abelson 2006), several studies have demonstrated the positive effect of artificial reefs that are used mainly for regulating diving and fishing pressures (Leeworthy et al. 2006; Polak and Shashar 2012). Thus, these artificial reefs help to alleviate this situation by allowing the degraded natural reefs to recover.
Deploying artificial reefs in degraded reef areas helps to restore the natural reefs by submerging artificial structures in proximity to them. These complex artificial structures promote the recruitment of reef organisms (e.g., corals, fish, sea-urchins) by adding shelter spaces and settlement substrates. Consequently, this leads to the building of a healthy reef that can provide a supply of both larvae (by recruitment) and adult (by migration) reef organisms to the degraded natural reef.
In many cases, reef degradation is the outcome of direct anthropogenic stressors, such as over-fishing, pollution, and recreational activities (Carilli et al. 2009). Thus, any restoration attempts will have limited success at best, if the stressors that cause the degradation are not mitigated (Abelson et al. 2016). The effects of anthropogenic stressors on recruitment are well known (Carilli et al. 2009), but at what stage of this succession process these effects actually start, remains unclear and requires further research.
The initial recruitment process to any substrate consists of the formation of a biofilm composed of diverse micro-organisms, which can already be found as early as 2 h following submersion of a new substrate (e.g., Janthinobacterium sp. and Stenotrophomonas sp.; Siboni et al. 2007; Crook et al. 2016). These organisms, which are mostly prokaryotes, adhere to each other and to the substrate to form a matrix (Bauer et al. 2002). Previous studies have revealed the influence of the biofilm (by means of physical and chemical cues), on the recruitment of macro-organisms in later successional stages of coral reefs (Wieczorek and Todd 1998; Webster et al. 2004). Most published examples describe crustose coralline algae (CCA) related biofilms and have attempted to isolate the biofilm components in order to determine which ones have an effect on recruitment (Negri et al. 2001; Siboni et al. 2020). One known example for a coral settlement chemical is the tetrabromopyrrole (TBP; Sneed et al. 2014). Another example, for a visual cue, is the red color (Mason et al. 2011). Additional cue types have been revealed, such as acoustic cues (Pysanczyn et al. 2023) and topographic cues (Whalan et al. 2015). Although the impact of the biofilm community on the characterization of the reefs is not yet fully understood, it is assumed to play an important role in that process (Ainsworth et al. 2010). It is, therefore, necessary to examine if and how the biofilm community is affected by environmental conditions. This is the aim of the current study.
Although many studies have examined biofilms on coral-reef substrates, most of them have only dealt with prokaryotic organisms. Unfortunately, the presence of eukaryotic organisms in early biofilm formation has been largely ignored, despite their potential role in the recruitment of macro-organisms in later stages. The current study explores the early successional stages (during the first 6 months) that occur on newly introduced artificial substrates deployed in coral reefs. Specifically, we aim to determine whether the initial biofilm is affected by environmental conditions (including anthropogenic stress), particularly community composition and successional dynamics of both prokaryotic and eukaryotic organisms.
Materials and methods
Study sites
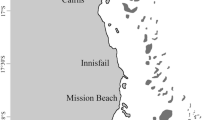
Two coral-reef sites along the shores of Eilat, Israel, were chosen for this study: the Dekel beach (N-29°32.387', E-34°56.784'; Fig. 1) and the IUI (N-29°30.141', E-34°55.040'; Fig. 1). The Dekel beach is a common tourist beach with a restaurant and a dive center. In addition to the many swimmers and divers that enter the water, the site is also located between the port and the naval base, which makes it a disturbed site (under heavy anthropogenic pressure). The site has a sandy bottom with a few large knoll reefs and a few old artificial structures. The IUI is a site off the shore of the Interuniversity Institute for Marine Science. It has limited access for swimmers and divers, is located just south of the protected underwater reserve, and is, therefore, considered to be a conserved site (under low anthropogenic pressure). The site has a bottom covered in small rocks with a relatively large continuous patch of reef and a few artificial structures.
The levels of nitrogen forms that can be considered as pollutants (ammonia and nitrate) were measured during the year of the experiment by the national monitoring program (NMP 2019), and the findings support the claim that the Dekel site is more anthropogenically affected than the IUI site. Since the two sites are not regularly surveyed by the NMP, the two closest monitored sites were chosen: the Japanese gardens in the nature reserve near the IUI and the naval base near the Dekel site, both of which are less than 500 m from the experimental sites (Fig. 1). The NMP takes water samples once a month. Our data covered the whole year of the experiment, including three months before and three months after (September 2017–September 2018), and were averaged to determine the mean levels of ammonia and nitrate at both sites.
Experimental design
Most marine biofilm studies use different versions of settlement tiles (e.g., Siboni et al. 2007; Erwin et al. 2008; Sawall et al. 2012; Sneed et al. 2014). In the present study, we use a novel method, which we termed ‘tab-by-tab’, and which has many advantages, as specified below.
At each of the two examined sites, a 20 cm, L-shaped HDPE pipe (110 mm dia.) was submerged and tied between a module and a buoy, suspended in the water, at a depth of 5 m (Fig. 2), approximately 3 m from the seabed, in December 2017. The module is a larger structure, comprising three vertically connected, HDPE pipes (110 mm dia.), 50 cm in length (Fig. 2). The purpose of the module is to attract both sessile and motile, reef-related macro-organisms (like fish, corals, etc.). The L-shaped pipes used for the experiment were cut around the edges prior to their submersion into equally sized tabs, still connected to the pipe at one end (Fig. 2). Each tab constituted a sampling unit, and at each sampling round, four tabs were sampled to serve as repetitions for statistical purposes. The tabs were of a size that could fit into the tubes of the DNA extraction kit. During sampling, the tabs were cut out with a knife while held with tweezers, and were then transferred directly into a sterile tube. Following collection, the samples were kept frozen at − 80 °C until the DNA extraction.
Working underwater while scuba-diving has some challenges, mainly, time limit and the inability to operate electrical tools. The ‘tab-by-tab’ method offers a quick and simple way to collect substrate samples, while also minimizing the physical damage to the biofilm by minimizing vibration, particle fluidization, and physical handling.
In addition to the tabs, water samples from the water column in vicinity of the structures were also taken. During each sampling round, two repetitions were collected using two 0.5L, sterilized plastic bottles. The bottles were then kept frozen in − 80 °C until they were processed whereupon they were thawed and filtered through 0.2 μm, sterilized filters. The filters were then used for DNA extraction. Following the DNA extraction, all samples were sent for sequencing of both eukaryotic (18S unit) and prokaryotic (16S unit) organisms.
Samples were taken four times: 24 h (December 2017), 1 month (January 2018), 4 months (April 2018), and 6 months (June 2018) following deployment. At each sampling time, four replicates of substrate samples and two of seawater samples were taken from each site. Some replicates were omitted due to a low number of reads. But all the samples consisted of at least two replicates.
Eight months after the experiment started (August 2018), the larger modules were surveyed for newly recruited corals (up to 1 cm in dia.) at both sites, using a blue flashlight during night dives which enabled observation of the coral’s fluorescence trait. Twelve months after the experiment had started (December 2018), the modules were surveyed again, this time for veteran recruits (larger than 1 cm in dia.) at both sites.
16S and 18S rRNA gene sequencing and analysis
Total genomic DNA was extracted from the samples using the MoBio PowerSoil DNA isolation kit (MoBio Laboratories. Inc., Carlsbad, CA, USA) following the protocol supplied by the manufacturer. An empty sample was tested with gel electrophoresis as a control to eliminate the option of lab-related environmental contaminations. The extracted DNA was submitted to the DNA Services Facility (DNAS), at the Research Resources Center, the University of Illinois at Chicago (UIC), for bacteria and eukaryote small subunit (16S and 18S, respectively) ribosomal RNA (rRNA) gene sequencing using the Illumina MiniSeq platform. Prior to sequencing, two steps of PCR amplification were performed. During the first PCR, fragments of the V4 region of the bacterial 16S rRNA and V9 region of the eukaryotic 18S rRNA gene were amplified using universal primers, used by the earth microbiome project (515F/806R for 16S and Euk1391F/EukBR for 18S; for sequences see section S6 in the supplemental material), attached with 5′ linker sequences, CS1, and CS2 (known as common sequence 1 and 2). An additional PCR reaction was performed for library preparation (Green et al. 2015).
Raw reads were merged using the software package PEAR v0.9.10 (Zhang et al. 2014). Low-quality sequences and chimeras were removed by the software package Mothur v1.36.1 (Schloss et al. 2009). The quality-controlled sequences were processed with the Quantitative Insights into Microbial Ecology (QIIME v1.9.1) package (Caporaso et al. 2010). Briefly, sequence data were clustered into operational taxonomic units (OTU) at 97% similarity using UCLUST. Representative sequences from each OTU were extracted and aligned using PyNAST with a percentage identity threshold of 90% to the Silva database.
Alpha and beta diversity, including the observed species index, the community richness (Chao1 index), Whole tree PD index, Shannon’s diversity index, and dominance index were calculated for all samples (Table S1 in supplemental material) using Quantitative Insights into Microbial Ecology (QIIME v1.9.1) package (Caporaso et al. 2010).
The phylogenetic data were used to assess the differences in relative abundance and diversity indices between the two sites.
Linear discriminant analysis effect size (LEfSe) analysis was performed for 16S samples to find biomarkers between the two sites, using the Galaxy web platform (Afgan et al. 2018) via the website: https://huttenhower.sph.harvard.edu/galaxy/. Samples were filtered for organisms above a relative abundance level of 0.1%, LDA was set to 4, and all alpha values were set to 0.05.
Statistical analysis
All statistical analyses were performed with R 3.0.3 (R Development Core Team R 2017) and α was set to 0.05. Permutation MANOVA and ANOSIM tests were performed for microbial communities testing, Permutation ANOVA tests were performed for coral recruitment testing, and paired t-tests were performed for diversity indices testing. All error-bars in the graphs represent standard errors. P values of permanova and ANOSIM tests are provided in Table S2 in supplemental material.
All statistical calculations were performed on the OTU level, yet the figures in the ‘results’ chapter show the phylum level for easier visualization purposes. For higher resolution (lower taxonomic level) of prokaryotes and eukaryotes, see Fig. 5 and Fig. S3 in supplemental material, respectively.
Results
Ammonia and nitrate
Data collected by the national monitoring program [28] revealed differences in ammonia and nitrate levels between the two sites (Ammonia (nmol/L): Dekel-171.7, IUI-94.7, Nitrate (nmol/L): Dekel-477.4, IUI-170.0; Fig. 1).
Microbial diversity
To study the microbial diversity, the 16S and 18S rRNA gene were sequenced from 48 substrate and water samples in total. Using a set of bioinformatic filters (as detailed in the ‘Methods’ chapter), we generated a sequence dataset containing high-quality data (3,068,951 and 2,558,683 screened reads were obtained from 48 samples for 16S and 18S samples, respectively). Rarefaction curves (Fig. S4 in supplemental material) reached saturation at around 20,000 and 6000 reads per sample for the 16S and 18S samples, respectively, indicating that the sequencing depth was roughly enough to cover the relatively large diversity (Table S1 in supplemental material).
16S rRNA gene analysis
The microbial communities in the water column from the Dekel beach site and the IUI site revealed no significant differences between them, for all four sampling rounds (Permanova test, see p-values in Table S2 in supplemental material). In contrast, the substrates did reveal a significant difference between the two sites, for all sampling rounds (Permamova test, see p-values in Table S2 in supplemental material), except for the 24 h sample (Permanova test, see p-value in Table S2 in supplemental material). The differences between sampling rounds were also significant for the two sites, on both the substrate, and in the water (Permamova test, pdek-sub = 0.001, pdek-wat = 0.017, piui-sub = 0.001, piui-wat = 0.015). Changes in community composition on the substrates could be observed even at the phylum level (Figs. 3 and 4) and on lower taxonomical levels (Fig. 5). Twenty-four hours post deployment, the two most abundant phyla at both sites were the Proteobacteria and the Bacteroidetes, which comprised more than 60% of the community. While the abundance of Proteobacteria continued to increase at both sites (to about 60% after 6 months), the abundance of Bacteroidetes decreased after 1 month and thereafter remained relatively stable. Planctomycetes showed a high relative abundance after 24 h (7–11%), which dropped after 1 month (to 3–4%) and remained relatively stable until the end of the experiment.
Heatmap of Prokaryotic (16S gene) relative abundance at the different sites and sampling rounds, on a genus level (when possible), after filtration of samples with under 1% relative abundance. The names of the samples are mentioned at the bottom and describe the sample. The first part of the name represents the site (Dekel/IUI), the second part represents the source of the sample (Seawater/Substrate of the structure), and the third part represents the sampling round time (24 h/1 month/4 months/6 months)
LEfSe results revealed a complex picture as every sampling round revealed different potential biomarkers between the substrates of the two sites (Fig. 6) when the LDA was set to 4 (Fig. S5 in supplemental material). For example, on the one-month sampling round, Cyanobacteria groups were biomarkers for the Dekel site; while on the six-month sampling round, they were biomarkers for the IUI site, and did not appear as potential biomarkers on the 4-month sampling round. The 24-h sampling round and the all the sampling rounds of the water samples revealed no potential biomarkers even when the LDA was set to 2.
The observed OTU numbers and estimated species richness (by Chao1 index) show higher numbers on the substrate, compared to the water (paired t-test, p = 0.0004 and 0.0007, respectively; Table S1 in supplemental material). In addition, both the Shannon's diversity index and the PD whole tree index give higher scores to the substrate, as opposed to the Dominance index (paired t-test, p < 0.0001, 0.0025, and < 0.0001, respectively; Table S1 in supplemental material).
18S rRNA gene analysis
The eukaryotic taxa revealed no meaningful results of ecological functions. Therefore, we focused on specific groups of ecological interest. The full presentation of the relative abundance measures can be found in the supplemental material (Fig. S3).
All the scores of the examined indices were lower compared to the prokaryotes (paired t-test, p < 0.0001 for all) except for the dominance which was higher in the eukaryotes (paired t-test, p = 0.0023; Table S1 in supplemental material).
In addition, the OTU numbers and Chao1 index scores show opposite trends to those revealed in the prokaryotes, as the eukaryotes had higher numbers in the water, compared to the substrate (paired t-test, p < 0.0001 for both; Table S1 in supplemental material).
The diversity indices showed an interesting result as the phylogenetic index (Whole tree) had no significant differences in scores between the water and substrate samples (paired t-test, p = 0.472), while the species index (Shannon’s) revealed higher scores in the water samples (paired t-test, p = 0.0176; Table S1 in s supplemental material).
The Dominance levels on the substrate showed a decrease with time. In the water samples, their levels increased significantly at the 6-month sampling round, and their scores were higher than those of the substrate samples (paired t-test, p = 0.0215; Table S1 in supplemental material).
The groups of interest that were chosen were the ones best reflecting reef health: subclasses Hexacorallia, Octocorallia, and Corallinophycidae (corals and coralline algae). The presented data are from the latest sampling round (after 6 months) when the communities were more stabilized. The IUI had higher average relative abundance of stony corals (Hexacorallia; 0.04% vs. 0%), soft corals (Octocorallia; 0.03% vs. 0%), and coralline algae (Corallinophycidae; 2.74% vs. 1.80%) compared to the Dekel.
Coral recruitment
Newly settled and veteran coral recruits surveyed on the modules during August 2018 and December 2018 showed significant differences between the IUI site and the Dekel sites (permutation ANOVA tests, p < 0.0001 in both cases), with roughly three times more coral recruits at the IUI (Fig. 7).
Discussion
The present study demonstrates that microbial community structure of biofilms on coral reefs may play an important role in dictating the reef’s community structure of corals and other sessile organisms. Different communities were formed on the substrates despite similar microbial communities in the water column of the two sites. This may indicate a potential role of different, site-specific, environmental conditions in determining the biofilm composition, leading to different paths of community development. In this way, site-specific, environmental conditions could determine biofilm composition, and consequently, could possibly play a role in the future development of the reef community.
Prokaryotic community
Our findings revealed that the planktonic prokaryotic communities at the two sites were similar throughout the sampling period, and so were the substrates in the 24-h sampling round of the study. We suggest that this can be explained by similar development in the initial colonizing stages. In contrast, a month after deployment, prokaryotic biofilm communities began to display significant differences. We speculate that this is the result of similar microbial communities in the water column at the different sites; subsequently, the divergence in the development of the communities is due to the different local environmental conditions. In other words, since this is essentially the same water mass, similar planktonic communities have dictated similar biofilm communities at the initial succession stages. Then, the different environmental conditions have dictated different biofilm community compositions by favoring some species over others.
Distinct biofilm communities are known to play a role in inducing different coral-reef assemblages (Ainsworth et al. 2010). Previous studies have revealed differences between biofilm communities at different sites which have been subjected to varying levels of anthropogenic stress (Kriwy and Uthicke 2011; Webster and Negri 2006; Kegler et al. 2017). Furthermore, differences have been described between the biofilm community and its surrounding bacterial community in the water column (Sweet et al. 2011). The findings of our study present similar results to those of Roik et al. (2016) and Ziegler et al. (2016) performed in the Red Sea, demonstrating the potential effect of anthropogenic stress on creating different biofilm communities. Our study provides further support to the notion that site-specific environmental conditions can determine the biofilm composition on coral-reef substrates regardless of the microbial community composition in the water column. Previous studies (e.g., Kriwy and Uthicke 2011) demonstrated how a change in water quality, following environmental conditions may lead to a change in the planktonic communities which, in turn, affect the biofilm community. Our study results reveal that the supply of key-species larvae to given reef sites may be insufficient to explain the difference between communities, as it is also heavily affected by the site-specific environmental conditions. That is, the trajectories of coral-reef communities are influenced by environmental conditions in more than one way and they start from the earliest stages of succession, during the biofilm formation. This conclusion matches those of previous studies that revealed the bacterial changes in response to environmental conditions (e.g., Glasl et al. 2019; Sawall et al. 2012; Witt et al. 2011, 2012), and even points out the influence on succession trajectories (e.g., Remple et al. 2021).
LEfSe results revealed different potential biomarkers at different sampling rounds which may point at changes in dynamics in developing microbial communities. Seasonality may be an additional factor that accounts for some of the changes as shown before (e.g., Glasl et al. 2020). Seasonal variables such as light penetration and nutrient levels may, in general, create favorable conditions for cyanobacteria and photosynthetic organisms during the winter months in the Dekel site, and during the summer months in the IUI site. Previous studies showed that the abundance of these photosynthetic organisms increases throughout the winter and peaks at the beginning of spring (March), and then decreases throughout the summer, until the next winter (Labiosa et al. 2003; Raitsos et al. 2013).
Other groups that revealed an interesting result were: (1) the Planctomycetes, that was revealed as a phylum that might play a role as early colonizers. (2) The SAR11 clade, which is a known, highly abundant, planktonic order (Morris et al. 2002) that appeared on the substrates. To the best of our knowledge, this is the first record describing this clade as part of a marine biofilm. (3) The hydrocarbon degraders (Cellvibrionalles) and the nitrogen fixers (Rhizobiales) were found as potential biomarkers for the IUI site and for the Dekel site, respectively, during the spring sampling.
Eukaryotic community
While Shannon’s diversity index yielded higher scores for the water samples, the PD whole tree did not. Therefore, although species diversity was higher in the water, no difference was observed regarding phylogenetic diversity. One explanation for this could be periodical events of temporal occurrence, such as gamete releasing or larvae hatching by a group of phylogenetically related organisms (e.g., corals, sponges, etc.). The dominance index levels on the substrate decreased with time which may point to a stabilization process of the community.
The IUI had higher abundance of stony corals, soft corals and coralline algae, all of which are indicators of a healthy, functioning reef (Littler and Littler 2007). While this notion can be rather intuitive for corals, coralline algae also play a major role in coral-reef ecology and its resilience, as described by Littler and Littler (2013): “As an indicator group they are functionally resilient and able to expedite the recovery and restoration of coral-reef systems relatively quickly since some thin forms of crustose coralline algae accelerate colonization and chemically attract and facilitate the survival of coral larvae.” The differences in abundance of these groups could be derived from varying sets of environmental conditions and if so, may reveal their impact on the early stages of succession.
Differences between prokaryotes and eukaryotes
Examining the number of OTUs found in the different samples (Table S1 in supplemental material) reveals some interesting points. All prokaryotic sequences showed an increase in numbers between December and January, and then a decline from January to June, signifying a peak in species richness during winter. In the eukaryotes, the water samples revealed a similar trend, while the substrate samples demonstrated different trends. This may indicate that there were no seasonal influences at the sites on the biofilm as compared to plankton. For the prokaryotes, there were more OTUs on the substrate at both sites, as opposed to the eukaryotes, for which there were more OTUs in the water. This interesting observation indicates that, in prokaryotes, less common and possibly seasonal or transient OTUs settled on the substrates, while in eukaryotes, more common OTUs settled there. This hypothesis is further supported by the richness and diversity indices (Chao1 and Shannon’s) and the dominance index (Table S1 in supplemental material). Thus, higher diversity and lower dominance levels can be found on the substrates (compared to in the water) in prokaryotes, and the exact opposite is true in eukaryotes (Table S1 in supplemental material).
Differences between the sites
The Dekel and the IUI sites are approximately 4.7 km apart, yet their reefs are exposed to very different environmental conditions, including higher anthropogenic activities at the Dekel site. Local anthropogenic stressors are well-known as coral recruitment-inhibiting factors in coral reefs (e.g., Abelson et al. 2016; Richmond et al. 2018). The Dekel site is located at a tourist beach, relatively close to the city of Eilat as well as the port and the naval base, while the IUI site is more remote and protected.
The current study revealed that both sites of Eilat reefs featured the same, seasonally related microbial community changes in the water, whereas different communities have developed on the substrates. These findings are in accordance with an earlier study, which describes a unique biofilm which developed independently of the surrounding planktonic community in other marine environments (Zhang et al. 2019). Although our study did not show any direct cause–effect associations to explain the differences between sites, it is suggested that environmental conditions (including anthropogenic stressors) may also affect the biofilm community development. The community on the substrates changed with time, probably representing the successional dynamics, along with seasonal changes. From a broader perspective, this succession is followed by macro-organisms, which eventually determine the reef’s characteristics. Tracing back to early succession stages may help us to detect the origins of coral-reef recruitment challenges.
Coral recruitment
As the main building organisms of the reef framework, corals and their recruitment are crucial and reliable measures for reef health and resilience (Yanovski and Abelson 2019). Our study results suggest different recruitment patterns at the two sites examined. We observed approximately three times higher recruitment rates and nearly twice the rates of adult corals on the structures at the IUI site, compared to the Dekel site.
The relationships between biofilm communities and coral recruitment may play an important role in coral-reef restoration efforts (Van Oppen et al. 2017). Previous studies showed connections between biofilm and macro-organism recruitment induced by physical and chemical cues (Wieczorek and Todd 1998). However, the exact relationships between the different biofilm communities and coral recruitment in our study sites are not fully understood, and remain to be determined.
Nevertheless, our results show that the abundance of the microbial families Halieaceae, Flavobacteriaceae, and Phormidiaceae, which were detected as biomarkers, are significantly higher on the substrates of the IUI, during the 4-month and 6-month sampling rounds. We suggest these families as potential taxa that can induce coral larval settlement. The first two were shown to be positively correlated with healthy coral abundance: the Halieaceae is a known coastal water family (Spring et al. 2015) that was revealed before as abundant in healthy parts of a diseased reef (Rosales et al. 2020). Some members of the Flavobacteriaceae can be found in at least 23 different coral taxa and were found to create antimicrobial compounds that inhibit the effects of known pathogenic species (Delgadillo-Ordoñez et al. 2022). The case for the Phormidiaceae is less clear as it is a cyanobacterial family, and it has no mentions in the literature in the context of corals and coral settlement.
Understanding these connections may contribute to the design of sound restoration plans. For example, adding a pre-submersion, conditioning stage for artificial reefs may help to attract a “preferred biofilm community” to create favorable conditions for the recruitment of reef organisms. Such a process may also facilitate the selection of ideal locations for artificial reefs based on local biofilm types.
A deeper understanding of biofilm recruitment and succession in coral reefs is crucial for future restoration efforts. The current study emphasizes the need to further study the effects of environmental conditions and anthropogenic stressors on coral reefs at the microbial level. This knowledge would provide us with a better grasp of the stressors’ effects, and would help to formulate new directions for mitigation and restoration efforts.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to use in other studies but they will become publicly available in the near future and are available from the corresponding author on reasonable request.
References
Abelson A (2006) Artificial reefs vs coral transplantation as restoration tools for mitigating coral reef deterioration: benefits, concerns, and proposed guidelines. Bull Mar Sci 78(1):151–159
Abelson A, Nelson PA, Edgar GJ, Shashar N, Reed DC, Belmaker J et al (2016) Expanding marine protected areas to include degraded coral reefs. Conserv Biol 30(6):1182–1191
Afgan E, Baker D, Batut B, Van Den Beek M, Bouvier D, Čech M et al (2018) The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 46(1):537–544
Ainsworth TD, Thurber RV, Gates RD (2010) The future of coral reefs: a microbial perspective. Trends Ecol Evol 25(4):233–240
Anthony K, Bay LK, Costanza R, Firn J, Gunn J, Harrison P et al (2017) New interventions are needed to save coral reefs. Nat Ecol Evol 1(10):1420–1422
Baine M (2001) Artificial reefs: a review of their design, application, management and performance. Ocean Coast Manag 44(3–4):241–259
Bauer TT, Torres A, Ferrer R, Heyer CM, Schultze-Werninghaus C, Rasche K (2002) Biofilm formation in endotracheal tubes. Association between pneumonia and the persistence of pathogens. Monaldi Arch Chest Dis 57(1):84–87
Bohnsack JA (1989) Are high densities of fishes at artificial reefs the result of habitat limitation or behavioral preference? Bull Mar Sci 44(2):631–645
Burke LM, Reytar K, Spalding M, Perry A (2011) Reefs at risk revisited. World Resources Institute, Washington, DC
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Meth 7(5):335
Carilli JE, Norris RD, Black BA, Walsh SM, McField M (2009) Local stressors reduce coral resilience to bleaching. PLoS One 4(7):e6324
Cinner J (2014) Coral reef livelihoods. Curr Opin Environ Sustain 7:65–71
Clark S, Edwards AJ (1999) An evaluation of artificial reef structures as tools for marine habitat rehabilitation in the Maldives. Aquat Conserv Mar Freshw Ecosyst 9(1):5–21
Costanza R, De Groot R, Sutton P, Van der Ploeg S, Anderson SJ, Kubiszewski I et al (2014) Changes in the global value of ecosystem services. Glob Environ Change 26:152–158
Crook ED, Kroeker KJ, Potts DC, Rebolledo-Vieyra M, Hernandez-Terrones LM, Paytan A (2016) Recruitment and succession in a tropical benthic community in response to in-situ ocean acidification. PLoS One 11(1):e0146707
Deáth G, Fabricius KE, Sweatman H, Puotinen M (2012) The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci 109(44):17995–17999
Delgadillo-Ordoñez N, Raimundo I, Barno AR, Osman EO, Villela H, Bennett-Smith M, Voolstra CR, Benzoni F, Peixoto RS (2022) Red Sea Atlas of coral-associated bacteria highlights common microbiome members and their distribution across environmental gradients—a systematic review. Microorganisms 10(12):2340
Erwin PM, Song B, Szmant AM (2008) Settlement behavior of Acropora palmata planulae: effects of biofilm age and crustose coralline algal cover. In: Proceedings of the 11th International coral reef symposium. Ft. Lauderdale, FL, p 1219–1223
Glasl B, Bourne DG, Frade PR, Thomas T, Schaffelke B, Webster NS (2019) Microbial indicators of environmental perturbations in coral reef ecosystems. Microbiome 7(1):1–13
Glasl B, Robbins S, Frade PR, Marangon E, Laffy PW, Bourne DG, Webster NS (2020) Comparative genome-centric analysis reveals seasonal variation in the function of coral reef microbiomes. ISME 14(6):1435–1450
Green SJ, Venkatramanan R, Naqib A (2015) Deconstructing the polymerase chain reaction: understanding and correcting bias associated with primer degeneracies and primer-template mismatches. PLoS One 10(5):e0128122
Heron SF, Eakin CM, Douvere F, Anderson KL, Day JC, Geiger E et al (2017) Impacts of climate change on World Heritage coral reefs: a first global scientific assessment. UNESCO World Heritage Center, Paris
Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JB et al (2017) Coral reefs in the Anthropocene. Nature 546(7656):82–90
Kegler P, Kegler HF, Gärdes A, Ferse SC, Lukman M, Alfiansah YR, Hassenrück C, Kunzmann A (2017) Bacterial biofilm communities and coral larvae settlement at different levels of anthropogenic impact in the Spermonde Archipelago, Indonesia. Front Mar Sci 4:270
Knowlton N (2012) Iconic coral reef degraded despite substantial protection. Proc Natl Acad Sci 109(44):17734–17735
Kriwy P, Uthicke S (2011) Microbial diversity in marine biofilms along a water quality gradient on the Great Barrier Reef. Syst Appl Microbiol 34(2):116–126
Labiosa RG, Arrigo KR, Genin A, Monismith SG, van Dijken G (2003) The interplay between upwelling and deep convective mixing in determining the seasonal phytoplankton dynamics in the Gulf of Aqaba: evidence from SeaWiFS and MODIS. Limnol Oceanogr 48(6):2355–2368
Leeworthy VR, Maher T, Stone EA (2006) Can artificial reefs alter user pressure on adjacent natural reefs? Bull Mar Sci 78(1):29–38
Littler MM, Littler DS (2007) Assessment of coral reefs using herbivory/nutrient assays and indicator groups of benthic primary producers: a critical synthesis, proposed protocols, and critique of management strategies. Aquat Conserv 17(2):195–215
Littler MM, Littler DS (2013) The nature of crustose coralline algae and their interactions on reefs. In: Research and discoveries: the Revolution of Science through Scuba. Ergernian contributions to the marine sciences. p 199–212
Mason B, Beard M, Miller MW (2011) Coral larvae settle at a higher frequency on red surfaces. Coral Reefs 30:667–676
Morris RM, Rappé MS, Connon SA, Vergin KL, Siebold WA, Carlson CA et al (2002) SAR11 clade dominates ocean surface bacterioplankton communities. Nature 420(6917):806–810
Negri AP, Webster NS, Hill RT, Heyward AJ (2001) Metamorphosis of broadcast spawning corals in response to bacteria isolated from crustose algae. Mar Ecol Prog Ser 223:121–131
NMP (2019) Israel’s National Monitoring Program at the Gulf of Eilat. https://iui-eilat.huji.ac.il/Research/NMPMeteoData.aspx
Pickering H, Whitmarsh D (1997) Artificial reefs and fisheries exploitation: a review of the ‘attraction versus production debate, the influence of design and its significance for policy. Fish Res 31(1–2):39–59
Pickering H, Whitmarsh D, Jensen A (1999) Artificial reefs as a tool to aid rehabilitation of coastal ecosystems: investigating the potential. Mar Pollut Bull 37(8–12):505–514
Polak O, Shashar N (2012) Can a small artificial reef reduce diving pressure from a natural coral reef? Lessons learned from Eilat, Red Sea. Ocean Coast Manag 55:94–100
Pysanczyn JW, Williams EA, Brodrick E, Robert D, Craggs J, Marhaver KL, Simpson SD (2023) The role of acoustics within the sensory landscape of coral larval settlement. Front Mar Sci 10:1111599
R Development Core Team R (2017) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
Raitsos DE, Pradhan Y, Brewin RJ, Stenchikov G, Hoteit I (2013) Remote sensing the phytoplankton seasonal succession of the Red Sea. PLoS One 8(6):e64909
Remple KL, Silbiger NJ, Quinlan ZA, Fox MD, Kelly LW, Donahue MJ, Nelson CE (2021) Coral reef biofilm bacterial diversity and successional trajectories are structured by reef benthic organisms and shift under chronic nutrient enrichment. NPJ Biofilms Microbiomes 7(1):84
Richmond RH, Tisthammer KH, Spies NP (2018) The effects of anthropogenic stressors on reproduction and recruitment of corals and reef organisms. Front Mar Sci 5:226
Rinkevich B (2005) Conservation of coral reefs through active restoration measures: recent approaches and last decade progress. Environ Sci Technol 39(12):4333–4342
Roik A, Röthig T, Roder C, Ziegler M, Kremb SG, Voolstra CR (2016) Year-long monitoring of physico-chemical and biological variables provide a comparative baseline of coral reef functioning in the central Red Sea. PLoS One 11(11):e0163939
Rosales SM, Clark AS, Huebner LK, Ruzicka RR, Muller EM (2020) Rhodobacterales and Rhizobiales are associated with stony coral tissue loss disease and its suspected sources of transmission. Front Microbiol 11:681
Sawall Y, Richter C, Ramette A (2012) Effects of eutrophication, seasonality and macrofouling on the diversity of bacterial biofilms in equatorial coral reefs. PLoS One 7(7):e39951
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Siboni N, Lidor M, Kramarsky-Winter E, Kushmaro A (2007) Conditioning film and initial biofilm formation on ceramics tiles in the marine environment. FEMS Microbiol Lett 274(1):24–29
Siboni N, Abrego D, Puill-Stephan E, King WL, Bourne DG, Raina JB, Seymour JR, Harder T (2020) Crustose coralline algae that promote coral larval settlement harbor distinct surface bacterial communities. Coral Reefs 39:1703–1713
Sneed JM, Sharp KH, Ritchie KB, Paul VJ (2014) The chemical cue tetrabromopyrrole from a biofilm bacterium induces settlement of multiple Caribbean corals. Proc R Soc B Biol Sci 281(1786):20133086
Spring S, Scheuner C, Göker M, Klenk HP (2015) A taxonomic framework for emerging groups of ecologically important marine gammaproteobacteria based on the reconstruction of evolutionary relationships using genome-scale data. Front Microbiol 6:281
Sweet MJ, Croquer A, Bythell JC (2011) Development of bacterial biofilms on artificial corals in comparison to surface-associated microbes of hard corals. PLoS One 6(6):e21195
Van Oppen MJ, Gates RD, Blackall LL, Cantin N, Chakravarti LJ, Chan WY, Putnam HM et al (2017) Shifting paradigms in restoration of the world’s coral reefs. Glob Change Biol 23(9):3437–3448
Webster NS, Negri AP (2006) Site-specific variation in Antarctic marine biofilms established on artificial surfaces. Environ Microbiol 8(7):1177–1190
Webster NS, Smith LD, Heyward AJ, Watts JE, Webb RI, Blackall LL et al (2004) Metamorphosis of a scleractinian coral in response to microbial biofilms. Appl Environ Microbiol 70(2):1213–1221
Whalan S, Abdul Wahab MA, Sprungala S, Poole AJ, de Nys R (2015) Larval settlement: the role of surface topography for sessile coral reef invertebrates. PLoS One 10(2):e0117675
Wieczorek SK, Todd CD (1998) Inhibition and facilitation of settlement of epifaunal marine invertebrate larvae by microbial biofilm cues. Biofouling 12(1–3):81–118
Witt V, Wild C, Anthony KR, Diaz-Pulido G, Uthicke S (2011) Effects of ocean acidification on microbial community composition of, and oxygen fluxes through, biofilms from the Great Barrier Reef. Environ Microbiol 13(11):2976–2989
Witt V, Wild C, Uthicke S (2012) Terrestrial runoff controls the bacterial community composition of biofilms along a water quality gradient in the Great Barrier Reef. Appl Environ Microbiol 78(21):7786–7791
Yanovski R, Abelson A (2019) Structural complexity enhancement as a potential coral-reef restoration tool. Ecol Eng 132:87–93
Zhang J, Kobert K, Flouri T, Stamatakis A (2014) PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30(5):614–620
Zhang W, Ding W, Li YX, Tam C, Bougouffa S, Wang R, Sun J et al (2019) Marine biofilms constitute a bank of hidden microbial diversity and functional potential. Nat Commun 10(1):517
Ziegler M, Roik A, Porter A, Zubier K, Mudarris MS, Ormond R et al (2016) Coral microbial community dynamics in response to anthropogenic impacts near a major city in the central Red Sea. Mar Pollut Bull 105(2):629–640
Acknowledgements
This project was funded by the Schulich Ocean Studies Center Initiative at Dalhousie University and was carried out in part at the Interuniversity Institute for Marine Sciences in Eilat.
We wish to express our gratitude to the national monitoring program for providing data; Prof. Eitan Ben-Dov and Prof. Nadav Shashar for their guidance and consultancy; Yotam Barr, Doron Ashkenazi, Adam Eshel, and Tzach Cohen for their help with the field work; and the Interuniversity Institute for Marine Sciences in Eilat for their help and assistance.
This paper is dedicated to the memory of our colleague and dear friend Dr. Itzchak Brickner, who was a big part of this study, but unfortunately could not see it being published.
Funding
Open access funding provided by Tel Aviv University. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
RY, AK, and AA established the study concept and design. RY and IB conducted the field surveys. RY and HB conducted the lab work and data analysis. All authors contributed in concept interpretations, manuscript writing, and editing. All the authors gave their final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This is an observational study. The XYZ Research Ethics Committee has confirmed that no ethical approval is required.
Additional information
Responsible Editor: C. Voolstra .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yanovski, R., Barak, H., Brickner, I. et al. The microbial community of coral reefs: biofilm composition on artificial substrates under different environmental conditions. Mar Biol 171, 74 (2024). https://doi.org/10.1007/s00227-024-04400-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04400-x