Abstract
Polar ecosystems are subjected to many stressors, including climate change, that impact their overall functioning. Seabirds are good bioindicators of these systems as they readily respond to changes in environmental conditions. To quantify how environmental changes affect their life history, data on seabird diet, spatial distribution and body condition are needed to reveal the underlying mechanisms. We explored possible drivers of the winter distribution of single-colony populations of Atlantic puffins Fratercula arctica and black-legged kittiwakes Rissa tridactyla, two of the most numerous seabird species in the North Atlantic. Based on carbon and nitrogen stable isotopic data from feathers moulted during winter migration, we identified three groups of Atlantic puffins and two groups of black-legged kittiwakes occupying different isotopic niches. We then used geolocator tracking data for the same birds to determine if these groups reflected parallel differences in the location of moulting grounds rather than differences in their diet. We found that the isotopic niches of the three Atlantic puffin groups likely resulted from their use of different habitats during winter moult. In contrast, the isotopic niches of the two black-legged kittiwake groups were more likely a result of differences in their diet, as both groups were distributed in the same area. These findings suggest that different winter feeding and/or migration strategies may play a role in shaping the body condition of individuals for their following breeding season. We discuss the role of environmental conditions encountered by seabirds during winter migration to further elucidate such intracolony divergence in strategies. As polar ecosystems experience rapid changes in environmental conditions, the approach presented here may provide valuable information for the development of effective conservation measures (taking both intra- and intercolony variability into account), and to better predict future impacts of climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Polar Regions, rapid climatic changes are triggering pronounced shifts and reorganizations in regional ecosystems (Ducklow et al. 2007; Moline et al. 2008). Seabirds have been increasingly studied in recent decades as they are proven good bioindicators of marine ecosystems health (Einoder 2009). However, seabirds are declining worldwide (Paleczny et al. 2015) and are now among the most threatened birds on Earth (Croxall et al. 2012; Dias et al. 2019). Some studies suggest that this may primarily be a consequence of excessive fisheries or a decrease in the availability of prey caused by changing environmental conditions (Grémillet and Boulinier 2009; Grémillet et al. 2018). However, for most migratory marine birds, limited knowledge of their distribution outside of the breeding season has limited investigations on the relationship between environmental conditions and population dynamics, through impacts on over-winter survival or on reproductive success via carry-over effects (Runge and Marra 2005; Ratikainen et al. 2008; Smith and Gaston 2012). Yet, the non-breeding period may well be crucial for seabird survival and fitness. It is therefore important to uncover key relationships between important life history traits and various environmental factors, such as sea surface temperature, weather conditions, primary production, fish stocks, and human activities that can affect the condition and ecology of seabirds. The need is particularly urgent in Polar Regions, which undergo rapid changes of their environmental conditions. This is required to better predict future population trends and to develop and implement appropriate conservation actions both locally and at larger scales.
The development of tracking technologies, such as miniaturized light-level geolocator tracking devices (GLS loggers), has revolutionized our knowledge of the non-breeding spatial range of migrant species, not the least for seabirds (e.g., Egevang et al. 2010; Frederiksen et al. 2012; Strøm et al. 2021). Attached to birds, these devices must be both light and ergonomic while having sufficient storage capacity to monitor the entire migration period. GLS loggers allow the collection of data providing information on broad migration patterns and behaviour. In addition, complementary approaches are needed to detail conditions at important staging and moulting areas, where the populations may be more susceptible to natural and human-induced stressors, and define and quantify how the seabirds interact with the environment.
Stable isotope ratios measured in bird tissues represent a complementary approach with GLS data. For example, analysing an inert tissue such as feathers can give indications on the diet and environmental conditions encountered by the birds during their regular feather moult events (Hedd et al. 2010), i.e., when groups of feathers are regrown after the old ones are shed. Depending on the species and the type of feathers considered, the moulting period may be spread over a few weeks to several months. This is a crucial time for seabirds, because moulting feathers may limit movements due to increased energetic cost of flight and diving, and result in decreased ability to avoid exposure to inclement weather conditions, including extreme events such as storms (Clairbaux et al. 2021; Reiertsen et al. 2021). By sampling feathers during the breeding season when the birds are onshore, we can access data on the conditions they experienced in the preceding non-breeding period when they were distributed far offshore and therefore inaccessible (Hedd et al. 2010). The δ15N values reflect the birds’ relative trophic position and are considered an indicator of diet composition during feather growth, while δ13C values mainly reflect their feeding habitat characteristics (i.e., temperature, coastal/offshore environment) (Kelly 2000). Thus, combining isotopic and spatial data is a complementary method to gather valuable information on the location (feeding and moulting grounds) and trophic interactions of seabirds during winter (Hedd et al. 2010; González-Solís et al. 2011; St. John Glew et al. 2019). Obtaining this type of information for many individuals sharing the same breeding site allows researchers to determine the degree of intracolony heterogeneity in migration paths and location of moulting grounds as well as the overall isotopic niche occupied by the colony (Jæger et al. 2009; St. John Glew et al. 2019). This can in turn be linked to carry-over effects on body condition and reproductive success for the individuals in a given colony (Fayet et al. 2017). Ultimately, a better understanding of the non-breeding part of the seabird life cycle can help identify main mechanisms behind negative demographic trends (Reiertsen et al. 2021).
Here, we investigate the over-winter movements and isotopic feather composition of Atlantic puffins Fratercula arctica (hereafter puffins) and black-legged kittiwakes Rissa tridactyla (hereafter kittiwakes) breeding at Røst in northern Norway. Puffins are one of the most abundant North Atlantic seabirds (~ 20 million individuals; Harris and Wanless 2011), but their population at Røst, which was known to be the largest colony in the world, has declined on average 3.1% per year from 1.4 million pairs in 1979 to 226000 pairs in 2022 (Anker-Nilssen and Røstad 1993, Anker-Nilssen and Øyan 1995, SEAPOP data portal at www.seapop.no), and puffins are now classified globally as ‘Vulnerable’ (IUCN 2018) and as ‘Endangered’ on the Norwegian Red List (Stokke et al. 2021). The kittiwake is also classified globally as ‘Vulnerable’ (IUCN 2019) and listed as ‘Endangered’ in mainland Norway (Stokke et al. 2021), where its breeding population has decreased by 6–8% per year since the mid-1990s (Barrett et al. 2006; Sandvik et al. 2014). The reasons for these population collapses are not fully understood, but a key factor is long-term depression of breeding success due to climate-induced scarcity of food in the colony areas (Anker-Nilssen 1992; Durant et al. 2003, 2006; Sandvik et al. 2014). Kittiwakes are also affected by increased predation from white-tailed eagles Haliaeetus albicilla (Anker-Nilssen et al. 2023). The conditions the birds encounter at other times of year may also have played a role but both species spend the winter offshore over large areas in the North Atlantic (Frederiksen et al. 2012; Fayet et al. 2017), which makes it extremely difficult to study them outside the breeding season. More information on their ecology during the winter is needed to fill an important gap in our current understanding of the life cycle of these species.
We looked at the inter and intraspecific variability of diets and migration routes for puffins and kittiwakes over a 3-year period by combining tracking data with isotopic composition of feather samples from the same birds. We hypothesised that within the same colony, different diet and/or migration strategies may be adopted by the birds, leading to different isotopic niches for groups of individuals. We aimed to (i) assess the potential of the approach to improve winter monitoring of the birds’ ecology, (ii) identify groups of individuals with different strategies in winter characterized by the location of their moulting grounds and/or feeding preferences, and (iii) explore the impact of these strategies on the birds’ body condition in relation to environmental conditions.
Materials and methods
Study area and data collection
Fieldwork was carried out at two study sites in the Røst archipelago, the southwestern tip of the Lofoten Islands in northern Norway: the puffin colony on the island of Hernyken (67°26′ N, 11°52′ E), and the kittiwake colony on the island of Kårøya (67°30′ N, 12°05′ E). To investigate spatial distribution and activity patterns during winter, breeding birds were captured on the nest (kittiwakes) using a noose pole or in mist nets erected on the colony surface (puffins) between May and August in 2015–2019 (Table 1). All were equipped with a GLS logger (1.0 g Migratetech® models C65 and F100, and 1.9 g Biotrack® model Mk4083, Cambridge, UK) fixed to an acrylic leg band. The added mass was always < 1% of the birds’ mean body mass (puffins 452 ± 29 g, kittiwakes 389 ± 38 g) to minimise potential impacts (Phillips et al. 2003; Vandenabeele et al. 2012). Body measurements including head + bill length, bill height at gonys and body mass, were taken at both capture and retrieval, and at one of these occasions, a small blood sample (< 50 μL) was collected from the metatarsal vein of each bird for molecular sexing. A total of 72 and 37 GLS loggers containing tracking data from the preceding winter (i.e., when the sampled feathers were grown) were retrieved from puffins and kittiwakes, respectively. During recapture, about ten feathers were collected from the cheek of each puffin and the back of each kittiwake for stable isotope analyses. These feathers are replaced in winter, and therefore assumed to be representative of the diet and feeding habitat of the birds during this period (Cherel et al. 2000). All birds were sexed by DNA from the blood samples stored in Lysis buffer at 5 °C, using the method described by Anker-Nilssen et al. (2017). We declare that the birds captured and handled for this study were treated according to the ethical framework for handling animals for research as defined by Norwegian legislation and with licence from the relevant authorities.
Analysis of tracking data
GLS provide two positions per day based on light levels with an accuracy of about 200 km (Phillips et al. 2004; Halpin et al. 2021). All GLS data were collected as part of the SEATRACK project (https://seapop.no/en/seatrack/) and processed following its standardised procedures (Bråthen et al. 2021). Latitude was estimated from day length and longitude from time of solar midnight/noon, following the method described in Lisovski et al. (2020). The positions used for analysis were smoothed twice by averaging consecutive noon–midnight or midnight–noon longitudes and latitudes. All locations from within the equinox periods (19 February–1 April, and 7 September–21 October) were filtered out as latitude cannot be defined accurately for these periods using GLS. Spatial analyses were performed using the packages oceanmap (Bauer 2017), sp (Bivand et al. 2013) and adehabitatHR (Calenge 2006) developed for the R statistical environment version 4.2.1 (2022-06-23) (R Development Core Team 2021). For the individuals for which several years of tracking data were available, only the data corresponding to the year of feather sampling were used. Winter spatial distribution of the birds during the estimated moult period was examined using kernel density analysis (Phillips et al. 2004). Kernels were calculated based on a grid size of 200 and on an extent of 0.2 of the grids. The 25% and 70% home range correspond to the smallest area on which the probability to relocate the animal is equal to 0.25 and 0.70, respectively. Kernel analyses were performed in a Mercator projection.
Analyses of stable isotopes
Isotopic analyses were conducted on three feathers from each individual (Jæger et al. 2009). When feathers were too small, one or two additional feathers were added to obtain a sufficient mass (~ 0.4 mg) for the stable isotope analyses. Prior to the analyses, each feather was cleaned to remove dirt and chemical external contamination using a 2:1 chloroform:methanol solution in an ultrasonic bath for 2 min, rinsed twice in a methanol solution, and dried for 48 h at 45 °C (Jæger et al. 2009). Stable isotope analyses were performed on ~ 0.5 mg of subsamples homogenized with scissors, whole feathers loaded into tin cups, and using an elemental analyser (Flash EA 1112, Thermo Fisher) coupled in continuous flow mode to an isotope ratio mass spectrometer (Delta V Advantage, Thermo Fisher, Bremen, Germany). Stable isotope abundances were expressed in δ notation as the deviation from standards in parts per thousand (‰), according to the conventional notation: δX = [(Rsample/Rstandard)− 1], where X is 13C or 15N and R is the corresponding ratio 13C/12C or 15N/14N. Standard values were Vienna Pee Dee Belemnite (VPDB) for C and atmospheric N2 (air) for N. Replicate measurements of internal laboratory standards (acetanilide) indicated that the measurement error was < 0.2% for both δ13C and δ15N values. Isotopic analyses were performed at the Littoral Environnement et Sociétés laboratory (LIENSs, La Rochelle, France).
Timing of moult
To relate isotopic composition of newly grown feathers with the environment, it is necessary to define the moulting period to allow to pinpoint bird location along GLS tracks. While there are some studies suggesting a time-range for the main moulting period of both puffins (Harris et al. 2014; Darby et al. 2022) and kittiwakes (Cramp and Simmons 1983), this period can vary between colonies as well as individuals (Anker-Nilssen et al. 2017). An attempt to define the moulting period of kittiwake back feathers was performed using GLS data on QGIS software version 3.4.13 (QGIS Development Team 2009). GLS tracks of individuals with extreme isotopic values compared to the mean value of the colony were investigated (> 1.5 standard deviation from the mean). We assumed that isotopic values reflect the habitat used by the birds during their moulting period; therefore, a bird with isotopic values different from the others had probably used a different habitat. In this way, with the support of GLS data, we can identify the period at which the individual has moved away from the other individuals in its colony. The period during which the individual track separated from the main pathway used by the other birds (23 November–7 January) was estimated to be the moult period of the kittiwake back feathers. The moulting period of puffin head feathers (22 January–19 February) was estimated following the same procedure.
Definition of bird groups and ecoregions
Hierarchical clustering analyses were performed on bird isotopic values (δ15N and δ13C) using the R packages cluster (Maechler et al. 2022), eclust (Bhatnagar 2017), and factoextra (Kassambara and Mundt 2020). For each species, Euclidean distance was calculated based on the isotopic data. The clustering of the data was performed following the agglomerative method Ward.D2. Based on the NbClust() R function, we chose to keep three clusters for puffins and two clusters for kittiwakes. The spatial distributions of clusters were considered as ecoregions (three distinct ecoregions for the puffins and one common ecoregion for the kittiwakes, see Results). Environmental conditions within the ecoregions were characterized by calculating the centroids of the 70% location density kernels for each ecoregion and averaging the environmental parameters over a 300 × 300 km square centred around each centroid. These areas were used to describe the environmental conditions encountered by most individuals of both species during their moulting period. For each ecoregion, the monthly averages of sea surface temperature, mixed layer depth and wind speed, all commonly used parameters to describe environmental conditions in seabird habitats offshore (e.g., Orben et al. 2014), were calculated for February. Data were retrieved from different open access sources proposed by the Copernicus platform (http://marine.copernicus.eu/). In addition, average chlorophyll-a concentration data from April to September of the previous year were obtained from the Oceancolour platform (https://www.oceancolour.org) and used as an index for the level of primary production during the last productive season (see Supplementary Information).
Isotopic niche width and overlap
To investigate interindividual variability in diet and location of moulting grounds, it was necessary to define the characteristics of the isotopic niches occupied by each group. Bayesian Layman's isotope metrics δ15N range and δ13C range, indicators of trophic diversity and niche diversification, respectively (Layman et al. 2007), were estimated for each group of puffins and kittiwakes using Stable Isotope Bayesian Ellipses in R with the SIBER package (Jackson et al. 2011). The isotopic niches of each of these groups were estimated using calculated convex hull areas and generating bivariate ellipses in SIBER (Jackson et al. 2011). Standard ellipses corrected for small sample size (SEAc) were estimated as a measure of the mean core group isotopic niche for each cluster. The overlap area between paired SEAc and convex hulls was calculated, and the respective percentage of overlap area was estimated for each group. Additionally, the Bayesian standard ellipse areas (SEAB) were also calculated to obtain unbiased estimates of the isotopic niche widths with credibility intervals (Jackson et al. 2011). Convex hull, SEAc, and SEAB were expressed in ‰2 (surface of isotopic niches).
Body condition index
Body condition of puffins was estimated using the scaled mass index (SMI) method, based on body mass and morphometric measurements from tag recovery. SMI was chosen as body condition index for puffins, because it is a better indicator of the relative size of energy reserves and other body components than ordinary least-squares residuals (Peig and Green 2009). To avoid bias due to sexual dimorphism, SMI was calculated separately for females and males. Among the linear body measurements, the head + bill length (L) showed the strongest correlation with body mass (M) on a log–log scale and was selected to calculate the SMI for puffins (males: R2 = 0.15, p = 0.028; females R2 = 0.40, p < 0.001). The calculation of the body condition index was performed using the R package lmodel2 (Legendre 2018). First, a bivariate plot of M versus L was produced to identify points that may skew the relationship. The potential outliers were identified using the function outlierTest() and influenceIndexPlot() and were removed. The best-fit line on the remaining points was obtained by the standardised major axis (SMA) regression on ln-transformed data. The slope of this regression gave the bSMA value used in Eq. (1). Then, the SMI was calculated for each individual (including outliers) following Eq. (1) (Peig and Green 2009)
where Mi and Li are the body mass and the linear body measurement of individual i, respectively; bSMA is the scaling exponent estimated by the SMA regression of M on L; L0 is the arithmetic mean of the L value for all individuals; and \({\widehat{M}}_{i}\) is the predicted body mass for individual i when the linear body measure is standardised to L0. We did not find any relationship between kittiwake body mass and their head + bill or bill depth for either males or females (males: p = 0.88 and p = 0.28, respectively; females: p = 0.86 and p = 0.95, respectively). The body mass of kittiwakes was therefore used as an estimator of their body condition instead of SMI. To compare body condition between bird groups, one-factor ANOVA tests were performed for the comparison of the mean when the assumptions were met. Otherwise, a non-parametric Kruskal–Wallis test was performed. All statistical analyses were performed using R software version 4.2.1 (2022-06-23) (R Development Core Team 2021).
Results
The dataset included 72 puffins (38 females and 34 males) and 37 kittiwakes (18 females and 19 males) (Table 1). To avoid potential biases due to annual variation or sex-related differences in stable isotope values, mean values of δ15N and δ13C were compared between years and between sexes for both puffins and kittiwakes using one-factor ANOVA test (Table 2). None of the means were found to be statistically different, considering both year and sex (p values ranging from 0.826 to 0.974 for puffins and from 0.059 to 0.649 for kittiwakes). Consequently, data from all years and both sexes were pooled in subsequent analyses.
Differences in stable isotopic data
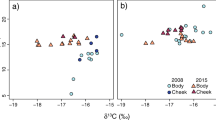
Hierarchical clustering analyses based on δ15N and δ13C isotopic values of puffins and kittiwakes, revealed three clusters for puffins and two clusters for kittiwakes. These clusters separate between groups of individuals occupying similar isotopic niches and are hereafter referred to as a group (Fig. 1). The results characterise these groups of individuals and reflect whether they resulted from a similarity in dietary and/or migratory strategies. For puffins, the mean δ15N value for group 2 (15.2‰ ± SD = 0.45) was higher than for groups 1 (13.5 ± 0.64) and 3 (13.8 ± 0.38), whereas the corresponding value for δ13C was higher for group 3 (−18.1% ± 0.25) than for groups 1 (−18.8 ± 0.21) and 2 (−18.8 ± 0.40). For kittiwakes, group 2 had higher δ15N and lower δ13C isotopic values (15.2 ± 0.55 and −19.3 ± 0.38) than group 1 (14.4 ± 0.43 and −18.7 ± 0.37). Among the puffin groups, the Bayesian Layman's range value for δ13C was highest for group 2 (1.46‰), whereas that for δ15N was highest for group 1 (2.60‰). Among the kittiwake groups, the Bayesian Layman's range values for both δ13C and δ15N were highest for individuals from group 2 (1.43 and 2.01, respectively). Puffin isotopic niche area, represented by convex hulls and both SEAC and SEAB, was larger for group 2 than for the two other groups. Group 2 of kittiwakes showed an isotopic niche area larger than that of group 1 (Table 3). For both species, no overlap in SEAC and convex hulls was found between the groups. Of the puffin groups’ trophic niche areas represented by SEAB posterior ellipses, 91% and 97% were higher for group 2 than for group 1 and 3, respectively, and most of the kittiwake groups’ SEAB posterior ellipses were higher for group 2 than for group 1 (92%) (Table 4).
The δ13C and δ15N isotopic niches of groups 1 (n = 31), 2 (n = 23) and 3 (n = 18) of Atlantic puffins (A) and of groups 1 (n = 14) and 2 (n = 23) of black-legged kittiwakes (B) from the Røst colony (Norway) during the estimated moulting period (puffins: 22 January–19 February; kittiwakes: 23 November–7 January). The solid-coloured lines enclose the standard ellipse area (SEAc) and the dotted coloured lines enclose the convex hull area for each cluster estimated by SIBER analysis (Stable Isotope Bayesian Ellipses in R, Jackson et al. 2011)
Differences in migration strategies
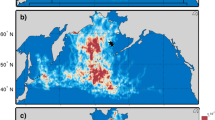
The δ15N and δ13C isotopic values obtained from feather samples correspond to the environmental conditions experienced by the birds, as well as their trophic interactions, while they were growing feathers (i.e., during the moulting period). For each group, the estimated location of the individuals during the moulting period was plotted based on GLS data (Fig. 2). Interestingly, this revealed consistent distribution patterns with variation in the moulting grounds for the three groups of puffins corresponding to broad ecoregions. Group 1 consisted of individuals distributed in the East Iceland (EI) ecoregion, group 2 the West Norwegian Sea (WNS) ecoregion, and group 3 the South Iceland (SI) ecoregion. Conversely, both kittiwake group 1 and 2 were found to be distributed within the same area, the East Labrador Sea (ELS). A higher proportion of the tracked kittiwakes were associated with group 2 (Table 5).
The 25% (dark shades) and 70% (light shades) kernel density ranges depicting the spatial distribution of birds in the isotopic groups (Fig. 1, Table 2) using data collected from geolocators attached to Atlantic puffins (A) and black-legged kittiwakes (B) from each ecoregion East Iceland (group 1 of puffins), West Norwegian Sea (group 2 of puffins), South Iceland (group 3 of puffins), and East Labrador Sea (groups 1 and 2 of kittiwakes) during the estimated moulting period (puffins: 22 January–19 February; kittiwakes: 23 November–7 January). The scale is in km
Differences in physical condition
For both species, physical condition was significantly lower for female than for male birds (ANOVA: puffins p < 0.001, kittiwakes p < 0.0001). However, neither male nor female puffins showed any significant difference in SMI between groups (Kruskal–Wallis: males p = 0.948, females p = 0.556). Despite the large overlap between SMI distributions for the three groups, the mean SMI for females from group 2 (448 ± 25 g) was seemingly higher than those from groups 1 and 3 (435 ± 28 g and 435 ± 47 g, respectively), whereas mean SMIs for males from group 2 and 3 (474 ± 33 g and 474 ± 34 g, respectively) were somewhat higher than that for group 1 (467 ± 42 g). For kittiwakes, no significant difference was found in the body mass of females from the two groups (group 1: 375 ± 39 g, group 2: 372 ± 24 g; Kruskal–Wallis p = 0.851), but male kittiwakes from group 2 had a significantly higher body mass than those from group 1 (group 2: 448 ± 19 g, group 1: 424 ± 14 g; Kruskal–Wallis p < 0.005) (Fig. 3).
Scaled Mass Index as a breeding season body condition proxy (mean, 25% and 75% percentiles) for female (group 1: n = 6, group 2: n = 12) and male black-legged kittiwakes (group 1: n = 8, group 2: n = 11) and female (group 1: n = 14, group 2: n = 13, group 3: n = 11) and male Atlantic puffins (group 1: n = 17, group 2: n = 10, group 3: n = 7) from each isotopic group identified for birds from the Røst colony. Significant difference in mean body mass is marked with an asterisk (p < 0.005)
Discussion
Alarming rates of changing conditions in polar areas accompanied by drastic trends in seabirds’ demography and population development urge the scientific community to explore new ways of studying seabirds’ ecology (Doyle et al. 2020). This is especially true for their poorly documented ecology during the non-breeding season. To date, only a few studies have used geolocation and/or stable isotope data to investigate isotopic niches and migration pathways of puffins and kittiwakes during the non-breeding season (Hedd et al. 2010; González-Solís et al. 2011; St. John Glew et al. 2019). To our knowledge, our study is the first to combine these two types of data in an integrated approach that models the intraspecific spatial segregation of trophic niches. We identified contrasting strategies adopted by individuals from the same colony with potential carry-over effects on their body condition in the following breeding season. Based on feather isotopic composition, reflecting conditions at the time and location they were grown, we identified groups of birds with different isotopic niches. These isotopic variations between groups can be the result of changes in diet (with groups feeding on prey of different trophic levels), or changes in the isotopic baseline, with groups exploiting different areas. Considering the latter, if the birds from the same colony have different wintering grounds, the food webs associated with these feeding areas might have different isotopic baselines (Rounick and Winterbourn 1986; Zohary et al. 1994; Cabana and Rasmussen 1996; Vander Zanden and Rasmussen 1999; Post 2002; Kjeldgaard et al. 2021). Thus, the birds from different groups could share a similar diet, but the differences observed between the isotopic niches could be due to an “isoscape effect”, i.e., spatial variation in the isotopic baseline the prey depends on (Barnes et al. 2009; Jennings and Warr 2003). For both species, the isotopic niches of each group revealed significant variations in strategies among individual during winter. For puffins, the three groups identified corresponded to different feeding grounds, leaving the possibility of variations in diet and/or isotopic baselines. For kittiwakes, GLS data showed that all individuals shared the same feeding grounds, implying that the difference in isotopic niches between the two groups was primarily explained by different dietary strategies.
Intracolony variability of strategies during winter moult
Previous studies on puffins have documented considerable intercolony differences in migration strategy (Harris et al. 2010; Guilford et al. 2011; Jessopp et al. 2013; Fayet et al. 2017). In this study, we highlighted intracolony variation and identified three diverging groups distributed over different areas, albeit with some spatial overlap. As mentioned before, the separation in isotopic niches of the three puffin groups could be due either to a change in the isotopic baselines between the ecoregions or a difference in diet. Because of the lack of isotopic data at large spatial scales with spatial resolution allowing to contrast the three ecoregions, we cannot assess with certainty which of the drivers is dominant. However, looking at δ15N and δ13C values separately can provide some clues on main drivers of isotopic variation between groups. Based on δ15N values, the birds in group 2 seemed to feed at a higher trophic level than those in the other two groups. Such differences in feeding behaviour might be related to seasonal fluctuations in prey abundance and/or differential energetic constraints during winter (Hedd et al. 2010). On the other hand, based on δ13C values, the isotopic difference between group 2 and 3 seemed to be determined by the environment they exploited.
In contrast to puffins, the GLS data revealed that the two kittiwake groups were distributed in the same area, and thus shared the same isotopic baseline. The distinction between their isotopic niches thus likely reflected differences in feeding strategies. Some previous studies have suggested that kittiwake diet can be composed of zooplankton (Aebischer et al. 1990; Dragańska-Deja et al. 2020). Given that around 85% of all North Atlantic kittiwakes winter west of the Mid-Atlantic Ridge (Frederiksen et al. 2012), increased competition for food resources could cause a trophic shift with the least competitive individuals relying on smaller prey. This would suggest that kittiwakes from group 1 that fed on a lower trophic level and therefore probably to a larger extent relied on small prey were at higher risk to jeopardize their physical condition. This hypothesise could explain why the body condition in the following breeding season of males from group 1 was lower than for those from group 2. Despite such differences (as discussed further below), this latest suggestion will easily be obscured by difference in the timing and breeding performance of the birds at sampling that also affects their body mass (Barrett et al. 1985).
Impacts of strategies on body condition
Like most seabirds, puffins and kittiwakes are extremely mobile organisms, suggesting a high metabolic rate (Bennett and Harvey 1987; Ellis and Gabrielsen 2002). Energetic costs associated with migration of wintering periods play a significant role on birds’ body condition and survival (Møller 1994; Klaassen 2003). Although higher body mass implies higher energetic cost to move and forage, it also reduces heat loss and, thereby, overall energy demand (Anker-Nilssen et al. 2018). In both species, the migration and/or feeding strategies adopted by individuals could result in carry-over effects from the physical condition or success of individuals in the preceding breeding season, as has been demonstrated for kittiwakes by Bogdanova et al. (2011, 2017). By affecting body condition, the feeding and migratory strategies of puffins and kittiwakes outside the breeding season may also affect their later condition for breeding.
A previous study conducted at large scale showed that puffin feeding and migration strategies in the non-breeding season are important determinants for the body condition and reproductive success of breeding individuals (Fayet et al. 2017). Based on our datasets, we did not detect any significant effect of the location of the winter moulting grounds on the birds’ body condition in the following breeding season. Potentially, this is again due to limitations associated with our dataset. Particularly, the morphometric measurements used for calculating SMI were most often made in the late incubation or early chick-rearing period when the mass of individuals fluctuates significantly and may be 20 to 30% lower than during winter (Barrett et al. 1985; Anker-Nilssen et al. 2018). To better estimate the carry-over impacts of different winter strategies, body mass of individuals at their arrival at the colony would be required.
For kittiwakes, the body condition of males was significantly related to their wintering (feeding) strategy, with those with a higher trophic status having a better body condition than those feeding on lower trophic level, probably involving smaller prey. Feeding on higher trophic levels would thus seem to be more favourable to mass gain, and possibly represent an advantage for reproduction following the winter period, as also shown for kittiwakes and three other seabird species breeding in Spitsbergen (Hovinen et al. 2019). The difference in diet could also reflect intraspecific competition for resources in winter. As the kittiwakes from Røst did not segregate spatially during this period, prey density was probably an important parameter likely to also affect the survival of adult kittiwakes during the non-breeding season, as shown for other colonies (Bogdanova et al. 2011; Reiertsen et al. 2014). Annual variations in the Barents Sea capelin stock and the abundance of Thecosomata sea snails off Newfoundland and Labrador, for example, can have an impact on the survival of Norwegian kittiwakes (Barrett et al. 2004; Reiertsen et al. 2014).
Influence of environmental conditions
The feeding and/or migration strategies adopted by seabirds outside the breeding season can be influenced both by biotic factors, such as food resources, and by abiotic factors in the physical environment they encounter during this period (Orben et al. 2015). Fluctuations in environmental conditions can impact the birds directly or indirectly. Direct impacts include extreme weather events, such as storms, which can result in seabird starvation with drastic impacts on seabird survival (Anker-Nilssen et al. 2017; Clairbaux et al. 2021). Higher temperatures also directly affect seabirds by increasing metabolic demands (Fort et al. 2009). On the other hand, changes in prey composition and/or quality can indirectly impact seabirds’ body condition. Lower trophic levels are known to be specifically sensitive to change in physical environmental conditions (Gaston et al. 2003), and changes in their abundance, community composition, or development timing, can readily influence higher trophic levels such as seabirds. The environmental conditions encountered by kittiwakes and puffins during the winter and migration periods could thus constrain the strategy adopted by an individual. The ecoregions EI, WNS, and SI identified in this study seem consistent with some of the ecological units proposed by Beaugrand et al. 2019. These ecological units were characterized by a specific combination of physical parameters and plankton composition/abundance, which could promote birds from a same population to develop divergent strategies. The WNS ecoregion is home of large copepod species such as Calanus hyperboreus (Choquet et al 2017), which can potentially lead to an increase in the abundance of higher trophic level food source for seabirds and could explain higher δ15N and SMI values for the puffins overwintering in this area. However, the relatively small size of our dataset in terms of number of birds sampled and temporal coverage limits our ability to identify the relationships between environmental conditions and the distribution and physical condition of the birds.
Potential of the isotopic approach in combination with GLS data
Based on the result of this study, we demonstrated that isotopic data are a useful tool to explore intracolony variability in winter strategies of seabirds. In combination with GLS data, we were able to link isotopic niches with ecoregions for three groups of puffins. A similar approach proved effective in explaining differences in contaminant concentrations between individuals from a single colony of red-legged kittiwakes (Fleishman et al. 2019), and this methodology has also potential to be extended further. Notably, it allows retrospective analysis by running stable isotope analysis on archived tissues (feathers) and in general to significantly increase the number of data collected every year (currently limited by recapturing birds equipped with GLS). Each bird could then be assigned to an ecoregion based on their stable isotope values. GLS data would, however, still be required to produce an original assessment of the moulting ground locations and associated isotopic niches (as done in this study), and to monitor any spatial/isotopic shifts in time.
Conclusion
Our study defines the location of offshore areas used by puffins and kittiwakes during their winter moult and, to the best of our knowledge, is the first to do so for black-legged kittiwakes. It is also one of the few to identify distinct differences in diet and/or migration strategies between groups of individuals from the same breeding population based on the complementary analysis of stable isotope and GLS data (Fleishman et al. 2019). Kittiwakes from the same colony showed some interindividual variation in their winter diet strategy, while puffins showed variation in their migration strategies by dispersing to different ecoregions. Further knowledge of isotopic baselines would be needed to improve our understanding of local scale effects, but the combined analysis of stable isotopic and GLS data provides a complementary view of the behavioural ecology of seabirds in winter. Differentiated strategy patterns as those we document here may prove to be more common in the future as anthropogenic pressures are expected to further impact the distribution of food resources and thus favouring niche partitioning to reduce interspecific and intraspecific competition. In addition, the increasing occurrences of extreme weather events due to fast climate change are expected to further challenge seabirds’ ability to survive. The conservation of Arctic seabirds will rely on local measures (including regulations of fisheries affecting key seabird prey) and global measures (to limit global warming), with a better understanding of seabird winter ecology being required for the former.
Data Availability
Stable isotopes data will be made available as supplementary material upon acceptance of the manuscript. To access GLS data, please get in touch with members of the SEATRACK project.
References
Aebischer NJ, Coulson JC, Colebrookl JM (1990) Parallel long-term trends across four marine trophic levels and weather. Nature 347:753–755. https://doi.org/10.1038/347753a0
Anker-Nilssen T (1992) Food supply as a determinant of reproduction and population development in Norwegian Puffins Fratercula arctica. Dr scient thesis, University of Trondheim, Norway
Anker-Nilssen T, Øyan HS (1995) Long-term studies of the breeding biology of puffins at Røst. NINA Fagrapport. 15:1–48
Anker-Nilssen T, Røstad OW (1993) Census and monitoring of puffins Fratercula arctica on Røst, N Norway, 1979–1988. Orn Scand 24:1–9. https://doi.org/10.2307/3676402
Anker-Nilssen T, Harris MP, Kleven O, Langset M (2017) Status, origin, and population level impacts of Atlantic puffins killed in a mass mortality event in southwest Norway early 2016. Seabird 30:1–14. https://doi.org/10.61350/sbj.30.1
Anker-Nilssen T, Jensen J-K, Harris MP (2018) Fit is fat: winter body mass of Atlantic puffins Fratercula arctica. Bird Stud 65:451–457. https://doi.org/10.1080/00063657.2018.1524452
Anker-Nilssen T, Fayet AL, Aarvak T (2023) Top-down control of a marine mesopredator: Increase in native white-tailed eagles accelerates the extinction of an endangered seabird population. J Appl Ecol 60:445–452. https://doi.org/10.1111/1365-2664.14343
Barnes C, Jennings S, Barry JT (2009) Environmental correlates of large-scale spatial variation in the δ13C of marine animals. Estuar Coast Shelf Sci 81:368–374. https://doi.org/10.1016/j.ecss.2008.11.011
Barrett RT, Fieler R, Anker-Nilssen T, Rikardsen F (1985) Measurements and weight changes of Norwegian adult puffins Fratercula arctica and kittiwakes Rissa tridactyla during the breeding season. Ringing Migr 6:102–112. https://doi.org/10.1080/03078698.1985.9673865
Barrett RT, Josefsen TD, Polder A (2004) Early spring wreck of black-legged kittiwakes Rissa tridactyla in north Norway, april 2003. Atl Seab. 6:33–46
Barrett RT, Lorentsen S-H, Anker-Nilssen T (2006) The status of breeding seabirds in mainland Norway. Atl Seab 8:97–126
Bauer R (2017) Oceanmap: mapping oceanographic data. R-Package Tutorial. https://doi.org/10.13140/RG.2.2.10079.59041
Beaugrand G, Edwards M, Hélaouët P (2019) An ecological partition of the Atlantic ocean and its adjacent seas. Prog Oceanogr 173:86–102. https://doi.org/10.1016/j.pocean.2019.02.014
Bennett PM, Harvey PH (1987) Active and resting metabolism in birds: allometry, phylogeny and ecology. J Zool 213:327–344. https://doi.org/10.1111/j.1469-7998.1987.tb03708.x
Bhatnagar SR (2017) eclust: Environment based clustering for interpretable predictive models in high dimensional data. R package version 0.1.0. https://cran.r-project.org/web/packages/eclust/eclust.pdf
Bivand R, Pebesma E, Gomez-Rubio V (2013) Applied spatial data analysis with R, 2nd edn. Springer, New York
Bogdanova MI, Daunt F, Newell M, Phillips RA, Harris MP, Wanless S (2011) Seasonal interactions in the black-legged kittiwake, Rissa tridactyla: links between breeding performance and winter distribution. Proc R Soc B 278:2412–2418. https://doi.org/10.1098/rspb.2010.2601
Bogdanova M, Butler A, Wanless S, Moe B et al (2017) Multi-colony tracking reveals spatio-temporal variation in carry-over effects between breeding success and winter movements in a pelagic seabird. Mar Ecol Prog Ser 578:167–181. https://doi.org/10.3354/meps12096
Bråthen VS, Moe B, Amélineau F, Ekker M (2021) An automated procedure (v2.0) to obtain positions from light-level geolocators in large-scale tracking of seabirds a method description for the SEATRACK project (NINA Report 1893). Norwegian Institute for Nature Research, Trondheim
Cabana G, Rasmussen JB (1996) Comparison of aquatic food chains using nitrogen isotopes. Proc Natl Acad Sci USA 93:10844–10847. https://doi.org/10.1073/pnas.93.20.10844
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Cherel Y, Hobson KA, Weimerskirch H (2000) Using stable-isotope analysis of feathers to distinguish moulting and breeding origins of seabirds. Oecologia 122:155–162. https://doi.org/10.1007/PL00008843
Choquet M, Hatlebakk M, Dhanasiri AKS, Kosobokova K, Smolina I, Søreide JE, Svensen C, Melle W, Kwaśniewski S, Eiane K, Daase M, Tverberg V, Skreslet S, Bucklin A, Hoarau G (2017) Genetics redraws pelagic biogeography of Calanus. Biol Lett 13:12. https://doi.org/10.1098/rsbl.2017.0588
Clairbaux M, Mathewson P, Porter W, Fort J, Strøm H, Moe B, Fauchald P, Descamps S, Helgason HH, Bråthen VS, Merkel B, Anker-Nilssen T, Bringsvor IS, Chastel O, Christensen-Dalsgaard S, Danielsen J, Daunt F, Dehnhard N, Erikstad KE, Ezhov A, Gavrilo M, Krasnov Y, Langset M, Lorentsen SH, Newell M, Olsen B, Reiertsen TK, Systad GH, Thórarinsson TL, Baran M, Diamond T, Fayet AL, Fitzsimmons MG, Frederiksen M, Gilchrist HG, Guilford T, Huffeldt NP, Jessopp M, Johansen KL, Kouwenberg AL, Linnebjerg JF, Major HL, Tranquilla LM, Mallory M, Merkel FR, Montevecchi W, Mosbech A, Petersen A, Grémillet D (2021) North Atlantic winter cyclones starve seabirds. Curr Biol 31:3964-3971.e3. https://doi.org/10.1016/j.cub.2021.06.059
Cramp S, Simmons KEL (1983) The Birds of the Western Palearctic Volume 3 Waders to Gulls. Oxford University Press, Oxford
Croxall JP, Butchart SHM, Lascelles B, Stattersfield AJ, Sullivan B, Symes A, Taylor P (2012) Seabird conservation status, threats and priority actions: a global assessment. Bird Conservation Int 22:1–34. https://doi.org/10.1017/S0959270912000020
Darby JH, Harris MP, Wanless S, Quinn JL, Brathen VS, Fayet AL, Clairbaux M, Hart T, Guilford T, Freeman R, Jessopp MJ (2022) A new biologging approach reveals unique flightless moult strategies of Atlantic puffins. Ecol Evol 12:e9579. https://doi.org/10.1002/ece3.9579
Dias MP, Martin R, Pearmain EJ, Burfield IJ, Small C, Phillips RA, Yates O, Lascelles B, Garcia Borboroglu P, Croxall JP (2019) Threats to seabirds: a global assessment. Biol Conserv 237:525–537. https://doi.org/10.1016/j.biocon.2019.06.033
Doyle S, Gray A, McMahon BJ (2020) Anthropogenic impacts on the demographics of Arctic-breeding birds. Polar Biol 43:1903–1945. https://doi.org/10.1007/s00300-020-02756-6
Dragańska-Deja K, Błaszczyk M, Deja K, Węsławski JM, Rodak J (2020) Tidewater glaciers as feeding spots for the black-legged kittiwake (Rissa tridactyla): a citizen science approach. Pol Polar Res 41:69–93. https://doi.org/10.24425/PPR.2020.132570
Ducklow HW, Baker K, Martinson DG, Quetin LB, Ross RM, Smith RC, Stammerjohn SE, Vernet M, Fraser W (2007) Marine pelagic ecosystems: the West Antarctic Peninsula. Phil Trans R Soc B 362:67–94. https://doi.org/10.1098/rstb.2006.1955
Durant JM, Anker-Nilssen T, Stenseth NC (2003) Trophic interactions under climate fluctuations: the Atlantic puffin as an example. Proc R Soc Lond B 270:1461–1466. https://doi.org/10.1098/rspb.2003.2397
Durant JM, Anker-Nilssen T, Stenseth NC (2006) Ocean climate prior to breeding affects the duration of the nestling period in the Atlantic puffin. Biol Lett 2:628–631. https://doi.org/10.1098/rsbl.2006.0520
Egevang C, Stenhouse IJ, Phillips RA, Petersen A, Fox JW, Silk JRD (2010) Tracking of Arctic terns Sterna paradisaea reveals longest animal migration. Proc Natl Acad Sci USA 107:2078–2081. https://doi.org/10.1073/pnas.0909493107
Einoder LD (2009) A review of the use of seabirds as indicators in fisheries and ecosystem management. Fish Res 95:6–13. https://doi.org/10.1016/j.fishres.2008.09.024
Ellis HI, Gabrielsen GW (2002) Energetics of free-ranging seabirds. In: Schreiber EA, Burger J (eds) Biology of Marine Birds. CRC Press, Boca Raton, pp 360–407
Fayet AL, Freeman R, Anker-Nilssen T, Diamond A, Erikstad KE, Fifield D, Fitzsimmons MG, Hansen ES, Harris MP, Jessopp M, Kouwenberg AL, Kress S, Mowat S, Perrins CM, Petersen A, Petersen IK, Reiertsen TK, Robertson GJ, Shannon P, Sigurðsson IA, Shoji A, Wanless S, Guilford T (2017) Ocean-wide drivers of migration strategies and their influence on population breeding performance in a declining seabird. Curr Biol 27:3871–3878. https://doi.org/10.1016/j.cub.2017.11.009
Fleishman AB, Orben RA, Kokubun N, Will A, Paredes R, Ackerman JT, Takahashi A, Kitaysky AS, Shaffer SA (2019) Wintering in the western subarctic Pacific increases mercury contamination of Red-Legged Kittiwakes. Environ Sci Technol 53:13398–13407
Fort J, Porter WP, Grémillet D (2009) Thermodynamic modelling predicts energetic bottleneck for seabirds wintering in the northwest Atlantic. J Exp Biol 212:2483–2490. https://doi.org/10.1242/jeb.032300
Frederiksen M, Moe B, Daunt F, Phillips RA, Barrett RT, Bogdanova MI, Boulinier T, Chardine JW, Chastel O, Chivers LS, Christensen-Dalsgaard S, Clément-Chastel C, Colhoun K, Freeman R, Gaston AJ, González-Solís J, Goutte A, Grémillet D, Guilford T, Jensen GH, Krasnov Y, Lorentsen SH, Mallory ML, Newell M, Olsen B, Shaw D, Steen H, Strøm H, Systad GH, Thórarinsson TL, Anker-Nilssen T (2012) Multicolony tracking reveals the winter distribution of a pelagic seabird on an ocean basin scale: winter distribution of Atlantic kittiwakes. Divers Distrib 18:530–542. https://doi.org/10.1111/j.1472-4642.2011.00864.x
Gaston AJ, Woo K, Hipfner JM (2003) trends in forage fish populations in northern hudson bay since 1981, as determined from the diet of nestling thick-billed murres, Uria lomvia. Arctic 56:227–233. https://doi.org/10.1443/arctic618
González-Solís J, Smyrli M, Militão T, Gremillet D, Tveraa T, Phillips RA, Boulinier T (2011) Combining stable isotope analyses and geolocation to reveal kittiwake migration. Mar Ecol Prog Ser 435:251–261. https://doi.org/10.3354/meps09233
Grémillet D, Boulinier T (2009) Spatial ecology and conservation of seabirds facing global climate change: a review. Mar Ecol Prog Ser 391:121–137. https://doi.org/10.3354/meps08212
Grémillet D, Ponchon A, Paleczny M, Palomares M-LD, Karpouzi V, Pauly D (2018) Persisting worldwide seabird-fishery competition despite seabird community decline. Curr Biol 28:4009–4013. https://doi.org/10.1016/j.cub.2018.10.051
Guilford T, Freeman R, Boyle D, Dean B, Kirk H, Phillips R, Perrins C (2011) A dispersive migration in the Atlantic puffin and its implications for migratory navigation. PLoS ONE 6:e21336. https://doi.org/10.1371/journal.pone.0021336
Halpin LR, Ross JD, Ramos R, Mott R, Carlile N, Golding N, Reyes-González JM, Militão T, De Felipe F, Zajková Z, Cruz-Flores M, Saldanha S, Morera-Pujol V, Navarro-Herrero L, Zango L, González-Solís J, Clarke RH (2021) Double-tagging scores of seabirds reveals that light-level geolocator accuracy is limited by species idiosyncrasies and equatorial solar profiles. Methods Ecol Evol 12:2243–2255. https://doi.org/10.1111/2041-210X.13698
Harris MP, Wanless S (2011) The Puffin. T & A D Poyser, London
Harris MP, Daunt F, Newell M, Phillips RA, Wanless S (2010) Wintering areas of adult Atlantic puffins Fratercula arctica from a North Sea colony as revealed by geolocation technology. Mar Biol 157:827–836. https://doi.org/10.1007/s00227-009-1365-0
Harris MP, Wanless S, Jensen J-K (2014) When are Atlantic puffins Fratercula arctica in the north sea and around the Faroe islands flightless? Bird Study 61:182–192. https://doi.org/10.1080/00063657.2014.909382
Hedd A, Fifield D, Burke C, Montevecchi W, McFarlane TL, Regular P, Buren A, Robertson G (2010) Seasonal shift in the foraging niche of Atlantic puffins Fratercula arctica revealed by stable isotope (δ15N and δ13C) analyses. Aquat Biol 9:13–22. https://doi.org/10.3354/ab00225
Hovinen JEH, Tarroux A, Ramírez F, Forero M, Descamps S (2019) Relationships between isotopic ratios, body condition and breeding success in a high Arctic seabird community. Mar Ecol Prog Ser 613:183–195. https://doi.org/10.3354/meps12886
IUCN (2018) The IUCN Red List of Threatened Species. IUCN Red List of Threatened Species, Gland
IUCN (2019) The IUCN Red List of Threatened Species. IUCN Red List of Threatened Species, Gland
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER - stable isotope bayesian ellipses in R: bayesian isotopic niche metrics. J Anim Ecol 80:595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x
Jæger I, Hop H, Gabrielsen GW (2009) Biomagnification of mercury in selected species from an Arctic marine food web in Svalbard. Sci Tot Environ 407:4744–4751. https://doi.org/10.1016/j.scitotenv.2009.04.004
Jennings S, Warr KJ (2003) Environmental correlates of large-scale spatial variation in the δ15N of marine animals. Mar Biol 142:1131–1140. https://doi.org/10.1007/s00227-003-1020-0
Jessopp MJ, Cronin M, Doyle TK, Wilson M, McQuatters-Gollop A, Newton S, Phillips RA (2013) Transatlantic migration by post-breeding puffins: a strategy to exploit a temporarily abundant food resource? Mar Biol 160:2755–2762. https://doi.org/10.1007/s00227-013-2268-7
Kassambara A, Mundt F (2020) Factoextra: extract and visualize the results of multivariate data analyses. R Package Version 1.0.7. https://CRAN.R-project.org/package=factoextra
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27. https://doi.org/10.1139/z99-165
Kjeldgaard MK, Hewlett JA, Eubanks MD (2021) Widespread variation in stable isotope trophic position estimates: patterns, causes, and potential consequences. Ecol Monogr 91(3):e01451. https://doi.org/10.1002/ecm.1451
Klaassen M (2003) Relationships between migration and breeding strategies in Arctic breeding birds. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian Migration. Springer, Berlin
Layman CA, Arrington DA, Montaña CG, Post DM (2007) Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88:42–48. https://doi.org/10.1890/0012-9658(2007)88[42:CSIRPF]2.0.CO;2
Legendre P (2018) lmodel2: model II regression. R Package. Version 1:7–3
Lisovski S, Bauer S, Briedis M, Davidson SC, Dhanjal-Adams KL, Hallworth MT, Karagicheva J, Meier CM, Merkel B, Ouwehand J, Pedersen L, Rakhimberdiev E, Roberto-Charron A, Seavy NE, Sumner MD, Taylor CM, Wotherspoon SJ, Bridge ES (2020) Light-level geolocator analyses: a user’s guide. J Anim Ecol 89:221–236
Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K (2022) cluster: Cluster analysis basics and extensions. R package version 2.1.4 — For new features, see the 'Changelog' file (in the package source). https://CRAN.R-project.org/package=cluster
Moline MA, Karnovsky NJ, Brown Z, Divoky GJ, Frazer TK, Jacoby CA, Torres JJ, Fraser WR (2008) High latitude changes in ice dynamics and their impact on polar marine ecosystems. Ann NY Acad Sci 1134:267–319. https://doi.org/10.1196/annals.1439.010
Møller AP (1994) Phenotype-dependent arrival time and its consequences in a migratory bird. Behav Ecol Sociobiol 35:115–122
Orben RA, Irons DB, Paredes R, Roby DD, Phillips RA, Shaffer SA (2014) North or south? niche separation of endemic red-legged kittiwakes and sympatric black-legged kittiwakes during their non-breeding migrations. J Biogeogr 42:401–412. https://doi.org/10.1111/jbi.12425
Paleczny M, Hammill E, Karpouzi V, Pauly D (2015) Population trend of the world’s monitored seabirds, 1950–2010. PLoS ONE 10:1–11. https://doi.org/10.1371/journal.pone.0129342
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891. https://doi.org/10.1111/j.1600-0706.2009.17643.x
Phillips RA, Wavier JC, Croxall JP (2003) Effects of satellite transmitters on albatrosses and petrels. Auk 120:1082–1090
Phillips RA, Silk JRD, Croxall JP, Afanasyev V, Briggs DR (2004) Accuracy of geolocation estimates for flying seabirds. Mar Ecol Prog Ser 266:265–272. https://doi.org/10.3354/meps266265
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods and assumptions. Ecology 83:703–718. https://doi.org/10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Ratikainen II, Gill JA, Gunnarsson TG, Sutherland WJ, Kokko H (2008) When density dependence is not instantaneous: theoretical developments and management implications. Ecol Lett 11:184–198. https://doi.org/10.1111/j.1461-0248.2007.01122.x
Reiertsen TK, Erikstad KE, Anker-Nilssen T, Barrett RT, Boulinier T, Frederiksen M, González-Solís J, Grémillet D, Johns D, Moe B, Ponchon A, Skern-Mauritzen M, Sandvik H, Yoccoz NG (2014) Prey density in non-breeding areas affects adult survival of black-legged kittiwakes Rissa tridactyla. Mar Ecol Prog Ser 509:289–302. https://doi.org/10.3354/meps10825
Reiertsen T, Layton-Matthews K, Erikstad KE, Hodges K (2021) Inter-population synchrony in adult survival and effects of climate and extreme weather in non-breeding areas of Atlantic puffins. Mar Ecol Prog Ser 676:219–231. https://doi.org/10.3354/meps13809
Rounick JS, Winterbourn MJ (1986) Stable carbon isotopes and carbon flow in ecosystems. Bioscience 36:171–177. https://doi.org/10.2307/1310304
Runge MC, Marra PP (2005) Modeling seasonal interactions in the population dynamics of migratory birds. Pop Ecol. 28:375–389
Sandvik H, Reiertsen TK, Erikstad KE, Anker-Nilssen T, Barrett RT, Lorentsen S-H, Systad GH, Myksvoll MS (2014) The decline of Norwegian kittiwake populations: modelling the role of ocean warming. Clim Res 60:91–102. https://doi.org/10.3354/cr01227
Seavy NE, Humple DL, Cormier RL, Gardali T (2012) Establishing the breeding provenance of a temperate-wintering north American passerine, the golden-crowned sparrow, using light-level geolocation. PLoS ONE 7:e34886. https://doi.org/10.1371/journal.pone.0034886
Smith PA, Gaston AJ (2012) Environmental variation and the demography and diet of thick-billed murres. Mar Ecol Prog Ser 454:237–249. https://doi.org/10.3354/meps09589
St john Glew K, Wanless S, Harris MP, Daunt F, Erikstad KE, Strøm H, Speakman JR, Kürten B, Trueman CN (2019) Sympatric Atlantic puffins and razorbills show contrasting responses to adverse marine conditions during winter foraging within the north sea. Mov Ecol 7:33. https://doi.org/10.1186/s40462-019-0174-4
Stokke BG, Dale S, Jacobsen K-O, Lislevand T, Solvang R, Strøm H (2021) Fugler Aves—Norge. In: ArtsdatabankenNorsk rødliste for arter 2021. Artsdatabanken, Trondheim
Strøm H, Descamps S, Ekker M, Fauchald P, Moe B (2021) Tracking the movements of north Atlantic seabirds: steps towards a better understanding of population dynamics and marine ecosystem conservation. Mar Ecol Prog Ser 676:97–116. https://doi.org/10.3354/meps13801
Vandenabeele SP, Shepard EL, Grogan A, Wilson RP (2012) When three per cent may not be three per cent; device-equipped seabirds experience variable flight constraints. Mar Biol 159:1–14. https://doi.org/10.1007/s00227-011-1784-6
Vander Zanden MJ, Rasmussen JB (1999) Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80:1395–1404. https://doi.org/10.1890/0012-9658(1999)080[1395:PCCANA]2.0.CO;2
Zohary T, Erez J, Gophen M, Berman-Frank I, Stiller M (1994) Seasonality of stable carbon isotopes within the pelagic food web of Lake Kinneret. Limnol Oceanogr 39:1030–1043. https://doi.org/10.4319/lo.1994.39.5.1030
Acknowledgements
The authors thank the reviewers, the plateforme Spectrométrie Isotopique of the Institut du Littoral, Environnement et Sociétés (LIENSs), Céline Albert and Gaël Guillou for the support with the stable isotope analyses. The deployment and retrieval of GLS-loggers and sampling of feathers were conducted as part of the SEATRACK project, made possible through close cooperation with the SEAPOP program (www.seapop.no, Norwegian Research Council grant #192141) and the ARCTOX network (https://arctox.cnrs.fr).
Funding
Open access funding provided by UiT The Arctic University of Norway (incl University Hospital of North Norway). This work was funded by SEAPOP program (Norwegian Research Council Grant #192,141).
Author information
Authors and Affiliations
Contributions
BE and TAN conceptualized the study together with JC who performed the analyses, produced the figures, and made the first draft under the supervision of BE and TAN. TAN led the collection of all data. All co-authors contributed to the final drafting of the manuscript and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The authors declare that all applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study have been followed and all necessary approvals have been obtained.
Additional information
Responsible Editor: V. Paiva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Charrier, J., Anker-Nilssen, T., Fort, J. et al. Intracolony variability in winter feeding and migration strategies of Atlantic puffins and black-legged kittiwakes. Mar Biol 171, 79 (2024). https://doi.org/10.1007/s00227-023-04384-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04384-0