Abstract
Investigating biological aspects of migratory marine animals like sea turtles is challenging. The Adriatic Sea is a key Mediterranean foraging ground for loggerhead turtles, yet certain feeding behaviors remain poorly known, including winter feeding and foraging within the neritic area of the Gulf of Manfredonia. With high fishing effort, this area experiences considerable turtle bycatch from trawlers during winter, representing an important conservation issue. Insights on how and to what extent the species interacts with anthropogenic threats such as trawlers and debris can be obtained from diet analysis. We examined feces and gut contents from 57 bycaught or stranded turtles to assess active feeding and digestion time during winter, identify and quantify prey items, evaluate feeding patterns among size classes, and ascertain the anthropogenic impact via debris and fishing discard ingestion. Our findings show that turtles feed actively during winter, primarily on benthic items, starting from a small size (32 cm Curved Carapace Length). No size effect was observed on dietary diversity or biomass percentage. We identified 37 Operational Taxonomic Units (OTUs) across 10 dietary groups, with crustaceans and mollusks being most prevalent. Osteichthyes and cephalopods, likely scavenged from trawl discard, were also common, implying intensive local trawling may attract turtles, increasing bycatch and mortality rates. The frequency of anthropogenic material ingestion was comparable to other Mediterranean regions and its presence in feces suggests non-lethal effects, if any. This study shows how information on seasonal feeding behavior can provide insights into how the relationship of the species with the environment and threats changes over time, ultimately steering conservation efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Investigating fundamental biological aspects is challenging in long-distance migratory marine animals—like sea turtles. For instance, the hypothesis of a discrete ontogenetic shift in sea turtles was prevalent, where small juveniles primarily fed within epipelagic zones until subsequently recruited to neritic, benthic foraging grounds (Bolten 2003). A more flexible foraging strategy has since been suggested, where turtles exploit both epipelagic and benthic habitats throughout their life stages (Hatase et al. 2006; Casale et al. 2008b; Cardona et al. 2010; Ramirez et al. 2017).
Feeding behavior affects the species at individual and population level, both in terms of growth and habitat use (Bjorndal et al. 2003). Diet and trophic role have been investigated directly through feces and gut contents (e.g. Limpus et al. 2001; Frick et al. 2009; Fukuoka et al. 2016), and stable isotopes (e.g. Wallace et al. 2009; Ceriani et al. 2017; Blasi et al. 2018; Haywood et al. 2020). Loggerhead turtles (Caretta caretta) are considered carnivorous generalists and favor invertebrates such as crustaceans and mollusks while feeding on the seafloor (Dodd 1988; Bjorndal 1997; Jones and Seminoff 2013), thus acting as bioturbators as they scour for food (Lazar et al. 2011). The presence of fish and cephalopods in the diet of loggerhead turtles has raised the question of the potential role of fishing discards in turtle diet (Tomas et al. 2001; Seney and Musick 2007). Turtles have limited ability to capture fast-moving and highly maneuverable prey items directly (Plotkin et al. 1993). In contrast, maneuverable species are targeted by fishing gears and end up being part of their discard, especially trawlers (Sánchez et al. 2007), becoming more easily accessible to turtles. Another source of concern is consumption of anthropogenic material, which can cause obstruction or internal lesions of the gastrointestinal tract, dietary dilution, and exposure to chemical contaminants (Derraik 2002; Senko et al. 2020).
In the Mediterranean Sea, loggerhead turtles (Caretta caretta) are the most abundant and widespread turtle species (Casale et al. 2018). They are protected both at national level and through international conventions (Casale et al. 2018). Their most important neritic foraging areas are the Adriatic Sea and the Tunisian Shelf, together with minor feeding areas in the Aegean Sea, East Turkey, Cyprus, Egypt, and Spain (Casale et al. 2018). An early recruitment to benthic foraging seems to be facilitated by the proximity of neritic and oceanic habitats across all these sites (Casale et al. 2008b).
The Adriatic Sea is a semi-closed basin with a wide latitudinal range. It is frequented only by loggerhead turtles of Mediterranean origin, especially from breeding sites in Greece (Tolve et al. 2018; Baldi et al. 2023). Here, turtles show a degree of fidelity to subareas (Casale and Simone 2017; Baldi et al. 2023) that may differ in trophic resources. So far, diet of loggerhead turtles in the Adriatic has been reported from the Central Adriatic (Mariani et al. 2023) and from the North Adriatic (Lazar et al. 2011), although limited to mollusks in the latter. To date there are no reports of diet from the Southern Adriatic and in particular from the Gulf of Manfredonia (GoM), recently identified as an important neritic area for the species (Casale et al. 2012a; Baldi et al. 2022). The bathymetry and environmental characteristics of the GoM (Marini et al. 2015) make it a suitable foraging area for sea turtles as well as a good fishing area for trawling (Casale et al. 2012a; Baldi et al. 2022). In fact, high sea turtle bycatch in this area has been reported, particularly during colder months and across a wide range of size classes (Casale et al. 2012a; Baldi et al. 2022), despite smaller turtles having reduced diving capacity (Hochscheid et al. 2007), thus potentially accessing less the benthic environment. If turtles were attracted to trawling discards, this would likely increase bycatch rates. This would have important conservation implications for both the juvenile turtles, which represent most of the population (Casale et al. 2012b), and the larger turtles, which have high reproductive value (Roast et al. 2023), ultimately affecting the Greek rookery from which most of these turtles originate (Tolve et al. 2018). The high bycatch reported during winter is of ecological interest, since Lazar et al. (2011) assumed that turtles do not feed in winter, at least in the cold waters of the north Adriatic, although direct evidence is still lacking.
This study aims to: (i) assess whether loggerhead turtles feed during winter; (ii) identify the prey items and their frequency; (iii) evaluate item preference patterns among size classes; and (iv) assess anthropogenic impact represented by the ingestion of debris and fishing discard.
Materials and methods
Sample collection
Samples were collected in the period from November 2019 to May 2021 when monthly mean Sea Surface Temperature (SST) averaged 15.0 ± 2.2 °C, vs. the other months when monthly mean SST was on average 23.8 ± 2.7 °C (Fig. 1, Table S2; OSTIA 2023). Loggerhead turtles incidentally caught by trawlers in the GoM in good body condition and without any external sign of illness were selected for the present study regardless of size. End location of hauls with capture events was provided by fishers. Freshly dead stranded loggerheads in good body condition were also collected for examination during the same time period. The Curved Carapace Length (CCL) was measured notch to tip (Bolten 1999). Live turtles were released at sea following the captivity period.
SST temperatures in the study period (Nov 2019–May 2021) in the Gulf of Manfredonia (41–42°N, 15.5–17°E). Data of the same months from different years have been pooled together. Data from OSTIA (2023)
Feces and gut content collection and examination
A previous study (Casale et al. 2008a) assessed a digestion period of three days during summer in southern Central Mediterranean. Since in the study area and period (winter) temperatures are more than 10 °C lower (OSTIA 2023), live turtles were kept in separate tanks for an observation period of ten days. If present, fecal samples were collected using a 0.4 mm mesh net and preserved in 70% ethanol until examination. Stranded or bycaught turtles found dead were examined by necropsy within hours after death or were stored frozen (− 18 °C) and then thawed before necropsy. Esophagus, stomach, and intestine contents were collected, if present, rinsed through a 0.4 mm mesh sieve with fresh water (Tomas et al. 2001) and stored in 70% ethanol.
At examination, the samples were rinsed in water to remove ethanol and drained through the same mesh. Identification of feces and gut contents was conducted with visual inspection for large fragments, or with a stereoscope at 20 × and 40 × magnification for small fragments. All samples were weighed (wet weight, ww) through a calibrated scale (Scale House model HLD150; accuracy 0.002 g). Then they were dehydrated in an oven at 60 °C for 48 h (Burke et al. 1993) to remove all residual water and weighed again (dry weight, dw). If the weight was larger than 0.002 g, prey items were sorted and assigned to the lowest possible taxonomic level through specific atlases (Riedl 1991; Giannuzzi-Savelli 1997; Giannuzzi-Savelli et al. 1999; Sabelli and Minelli 2009). In the 10 days of observation of live turtles, number of days from capture to first defecation, number and frequency of defecation were calculated.
Data analyses
All the analyses were performed in R (R Core Team 2023). To describe the diet composition of turtles, the following five variables were calculated: Frequency of Occurrence (FO), Taxa richness (TR), Simpson Diversity Index (SI), Dietary Groups (DG), Index of Relative Importance (IRI). Frequency of occurrence (FO) of each identified taxon was calculated as:
where Ty is the number of turtles with category y and T is the total turtles sampled. Taxa richness (TR) in the samples was calculated considering Operational Taxonomic Units (OTUs) (Sokal and Sneath 1963) of different taxonomic levels. Following Palmer et al. (2021), variation of taxa diversity with size was investigated with a negative binomial Generalized Linear Model (GLM) in the form TR ~ CCL, though the package MASS (Venables and Ripley 2002). Simpson Diversity Index’ values (SI; Simpson 1949) were calculated for each turtle that ingested at least one among the prey items as:
where pi is the proportional abundance of taxon i.
Then, their variation with size was analyzed with a binomial GLM in the form SI ~ CCL.
Taxa were grouped in Dietary Groups (DG) according to their ecological and morphological diversity: Vegetation (VEG—algae and seagrass), Gelatinous prey (GEL—Ctenophora and Tunicates), Porifera (POR), Mollusca (MOL), Crustacea (CRU), Echinodermata (ECH), and Osteichthyes (OST). Apart from biological items, the following groups were identified: indigestible (IND—wood and sediment), Anthropogenic material (AM), Not Assigned (N/A—anything that could not be sorted in any of the other categories; Table 1). The percentage of the Index of Relative Importance (IRI) was calculated for each following Bjorndal et al. (1997) and Howell and Shaver (2021):
where O is the proportion of the number of turtles with DGi on the total, and W is the proportion of the combined ww of the DG on the total ww (ww was chosen over dw as in the latter samples with high water content can be underrepresented). Pairwise Spearman’s rank correlations were performed to investigate correlations between anthropogenic debris and prey taxa.
DG weights were standardized by total weight of the sample per turtle (percent biomass per dietary group, BMDG) to account for differences in gastrointestinal capacity relative to turtles of different sizes (Echinoderms were excluded as they were found in only one turtle). Then, the effect of CCL on BM was investigated through a binomial family Generalized Additive Models (GAM) using the R-package mgcv (Wood 2011), in the form of BMDG ~ CCL.
Results
Feeding behavior
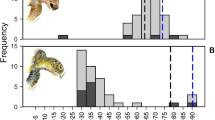
A total of 76 turtles were examined. Three turtles were found stranded dead, all had gut contents. 73 turtles were bycaught: 64 alive (48 defecated and 16 did not) and nine dead (six with gut contents and three without; Fig. 2). Hauls with bycaught turtles ended on average at 38.8 m (range = 18–54, SD = 14, n = 56). Most of the turtles were captured or stranded between November and March (n = 53), two in May, one each in September and October. One turtle was recaptured, and its feces items were pooled together in the analysis. The average size of the individuals that produced feces or gut contents was 62.5 cm CCL (SD = 11.2 cm; Range = 32.3–81.7 cm; n = 57). The mean number of defecations per turtle was 1.3 (SD = 0.7; Range = 1–4; n = 24) and the frequency was 0.13 day−1 (SD = 0.7 day−1; Range = 0.1–0.4 day−1; n = 24). Turtles first defecated on average after four days (SD = 2.8 days; Range = 0–9 days; n = 24) and until the 10th day in one case.
Mean wet weight (ww) of all the ingested items per turtle was 144.3 g (range = 0.05–2424.4 g; SD = 411.0 g, n = 57) and mean dry weight (dw) was 21.7 g (range = 0.01–352.0 g; SD = 51.8 g, n = 57), while mean wet weight (ww) of the prey items (animals only) per turtle was 85.9 g (range = 0.0–1744.3 g; SD = 274.6 g, n = 57) and mean dry weight (dw) was 16.9 g (range = 0.0–352.0 g; SD = 50.7 g, n = 57).
Diet composition
Only in few cases it was possible to identify the prey down to the species, six of which were not previously reported as prey items of the loggerhead turtle in the Mediterranean (Table 1). We identified 37 OTUs, 23 down to the family level and the other 14 comprising of taxa superior to family (Table 1). The range of taxa per turtle was 0–8 (mean = 2; SD = 2; n = 57), 0–6 (mean = 2; SD = 2; n = 48) for feces and 0–8 (mean = 3; SD = 3; n = 9) for gut contents. The Simpson diversity index ranged from 0.994 to 0.999 (mean = 0.998; SD = 0.001; n = 49). No significant difference was detected with change of size in TR (GLM, z = 0.10, p = 0.91) or SI (GLM, z = 0.10, p = 0.92; Fig. S1).
Of all Dietary Groups, VEG and POR had the highest %IRIfeces (n = 48), followed by CRU, although in a much lesser proportion (Table 2). The highest %IRIguts (n = 9) was found in GEL, followed by OST and POR and CRU with a lesser amount. In all %IRI, MOL represented a marginal contribution. ECH was found in only one turtle as sea urchins’ residues. Among Dietary Groups, the highest FO were CRU (40.4%), MOL (38.6%), and OST (29.8%; Table 1). Size did not show any significant effect on any DG (GAM, p > 0.05; Table S1; Fig. 3).
Anthropogenic material (AM) weighed on average 2.1 g per turtle (range = 0.02–16.2 g; SD = 4.32 g, n = 57). AM was found in 35.1% of the turtles, comprising of ropes, fragments of nets, and plastic sheets. No significant correlation was found between AM and other DG (ρ range = − 0.28–0.25, p > 0.05).
Discussion
This study provides the first assessment of loggerhead turtle’s diet in the large neritic foraging area of the south-western Adriatic (Gulf of Manfredonia). In doing so, it also provides the first evidence of active foraging during winter in the Adriatic.
Feeding behavior
At low temperatures, sea turtles are usually considered lethargic (Broderick et al. 2007). For this reason, in their study in the North Adriatic, Lazar et al. (2011) selected animals foraging in a warmer period assuming that they would be more active than a cold one. In our study area and during cold months, turtles displayed active feeding despite the low temperatures of waters of the GoM (Fig. 1), suggesting that most turtles remain active throughout the year (turtles were sighted at temperatures as low as 7 °C in the Atlantic; Epperly et al. 1995). This could be true for all or just a part of the population and could vary during the winter, as some turtles did not produce feces or gut contents. These turtles may have either defecated immediately at capture due to stress, had a long digestion, had a low feeding frequency or they were not feeding at all. In our study some turtles defecated for the first time up to the latest days of observation, which means that digestion time during winter is longer than what previously reported for warmer areas (Casale et al. 2008a). As expected by lower temperatures, average digestion time from capture to first defecation in our study (4 days) was longer than what was reported by Casale et al. (2008a) in warmer waters (2.5–3 days).
The results show that most individuals foraged on benthic prey, starting from a small size (min. 32 cm CCL). The shallow waters of the GoM (see Fig. 2) likely facilitate access to benthic prey to smaller turtles that have reduced diving capacity (Hochscheid et al. 2007). No effect of size was found on Taxa Richness and Simpson Diversity Index, and turtles that did not produce feces or gut contents spanned a wide size range, suggesting that individual variability in feeding behavior does not depend on size. Eleven individuals did not have a clear indication of benthic feeding (i.e. absence of sediment or other benthic-associated taxa; Tomas et al. 2001)—although for five of these, 100% of the material was Not Assigned (N/A).
Diet composition
Results indicate that turtles in the GoM display an opportunistic feeding behavior, as was reported in other areas in the Adriatic (Mariani et al. 2023) and other Mediterranean areas (Tomas et al. 2001; Casale et al. 2008b; Benhardouze et al. 2021; Palmer et al. 2021). Crustaceans had the highest FO, also found nearby in Central Adriatic by Mariani et al. (2023). In several cases, traces of the genus Squilla were found in our samples. Indeed, Squilla mantis occurs in the area and is a frequent target of trawlers (Baldi, unpub. data). Mollusks were the second most frequent Dietary Group found. Arca noae, Tonna galea and Fustiaria rubescens were never reported in other studies in the Mediterranean, while many other species, genera and superior taxa are shared with the North Adriatic (Lazar et al. 2011).
Gelatinous prey are likely underrepresented in our study since most of the samples came from feces and this item is likely easily digested. In support of that, GEL had among the highest IRIguts. For instance, Martin et al. (2021) did not find Ctenophores in gut contents but detected their presence only through DNA, supporting their fast digestion. Tomas et al. (2001) found a high frequency of Thaliacea in an area that is generally considered an oceanic area for small juveniles. In our study the two turtles with Thaliacea spanned different sizes and had other benthic items associated, which suggest variation in their habitat use regardless of their size and supports the relaxed ontogenetic shift suggested by Casale et al. (2008b).
Vegetation and Porifera were the taxa with the highest weight, due to one sample that contained > 2 kg of plant material and another that contained > 1 kg of sponges. These instances of a single turtle with a high weight contributed to the high IRI-total values and may not reflect population-level importance of these items in the diet." It is unclear why turtles would consume such a high quantity of these items, since they are not equipped to digest them (Laurent and Lescure 1994) and were in fact found largely undigested. Generally, it is thought that these items are incidentally ingested while foraging on other prey or looking for bacterial fauna or oligoelements in the case of sponges (Casale et al. 2008b; Palmer et al. 2021). The large amount observed here would support more the latter case than incidental ingestion. Chondrosia reniformis was the most frequently identified sponge and it was previously identified in trawl bycaught turtles (Casale et al. 2008b; Palmer et al. 2021).
Sea urchins were abundant in Casale et al. (2008b), but were only found once in our study, probably due to the differences in substrate. Other soft-bodied prey might have remained under-detected in our analyses, as seems to be suggested by the absence of rather common species in the marine habitat, for instance Cnidarians, Annelids or sea cucumbers, known to be present in the GoM (Storelli et al. 2001; De Leonardis et al. 2008) and present in the diet of loggerhead turtles in other areas (Tomas et al. 2001; Casale et al. 2008b; Benhardouze et al. 2021; Martin et al. 2021; Palmer et al. 2021). If turtles tend to frequent the trawled areas, the absence of some taxa could indicate depletion of benthic communities because of repeated trawling activity (Kaiser et al. 2003; Handley et al. 2014), i.e. reduction of biodiversity, substitution with more opportunistic or scavenger species.
Anthropogenic threats
Osteichthyes were the third most frequent taxa (29.8% FO), in four turtles they were over 80% of the total ww and OST had the highest IRIfeces. Together with Cephalopods, they are commonly considered to not be part of the natural diet of loggerhead turtles (Plotkin et al. 1993; Laurent and Lescure 1994). Fish presence in loggerhead diet studies is generally associated with fishing discard (Tomas et al. 2001; Seney and Musick 2007; Casale et al. 2008b; Benhardouze et al. 2021; Palmer et al. 2021). In support of that, Mullus barbatus, found for the first time in our study, is a common target of trawls (Baldi, unpub. data). These items suggest that turtles feed on fishing discard (Sánchez et al. 2007), a behavior reported by previous studies all over the basin through examination of feces and gut contents (Tomas et al. 2001; Seney and Musick 2007; Casale et al. 2008b; Benhardouze et al. 2021; Palmer et al. 2021) or stable isotope analysis (Blasi et al. 2018). In the Mediterranean, trawlers target multiple species (Lleonart and Maynou 2003) and produce large quantities of discard (Tsagarakis et al. 2014), making it available for consumption by turtles, or some of their prey (Mariani et al. 2023). This has important implications for conservation, since loggerhead turtles are also scavengers and may be attracted by fishing discard to areas with high fishing effort, increasing the probability to be incidentally captured. Such a concern calls for investigation about how fishing practices can be adjusted to reduce discard, which represents an objective for good fishing practices in general (Tsagarakis et al. 2014).
The present study reports frequency of anthropogenic material similar to Mariani et al. (2023) in the Central Adriatic and to Lazar and Gracan (2011) in the North, and other areas of the Mediterranean (Revelles et al. 2007; Casale et al. 2008b; Hochscheid et al. 2013; Palmer et al. 2021), but lower than other (Tomas et al. 2002; Solomando et al. 2022), suggesting a space–time variability in the distribution of debris or its consumption. The presence of debris in the feces demonstrates non-lethal effects of its ingestion. Loggerhead turtles seem to be more resistant to debris ingestion, which could be related to their feeding behavior, for instance they are less attracted to “floating” debris as a non-carnivorous turtle could be (Schuyler et al. 2014), that might be more prone to cause obstructions. Material found in our study was mostly small fragments, with one instance of one turtle with hook and line with a plastic bag attached. Although non-lethal ingestion is reported also in other studies (e.g. Tomas et al. 2002; Revelles et al. 2007; Casale et al. 2008b, 2016; Lazar and Gracan 2011), the effects of plastic consumption are not well understood and there are few long-term studies, especially on repeated intake over time. Prolonged presence in the digestive tract may increase the risks of internal injuries as well as sub-lethal effects, such as reduction of stomach capacity, inappetence, increased buoyancy, chemical contamination, which can lead to poor health and reduced fitness, if not death (Nelms et al. 2016) and references therein).
In the GoM, loggerhead turtles generally showed fidelity to the area (Casale and Simone 2017; Baldi et al. 2023), therefore dietary items identified in our sample can be considered representative of the prey available in the GoM. Most taxa can be found between 0 and 100 m depth, but some have a deeper range. Thus, is not easy to infer a specific depth preference at which turtles may prefer foraging from the prey items in our sample. It is possible that loggerhead turtles dived deep to feed, as reported in previous studies (Hatase et al. 2007; Narazaki et al. 2015), but some species might have been discarded by trawlers from deeper areas (Casale et al. 2008b). Nonetheless, the presence in most turtles of balanomorph barnacles on their carapace suggests a preference for shallower waters (Casale et al. 2004).
Conclusions
Feces collection has proven to be simple, noninvasive, and inexpensive, allowing for a larger sample size compared to gut collection, which requires dead animals, equipped infrastructures, and trained personnel. Samples collected in this way allow food contents to be obtained from healthy animals whose digestive functions are not impaired, providing representative samples of the diet. That is assuming that feces do not offer only a partial representation of the taxa biased towards the ones with hard parts, which does not seem to be the case from the present study and a previous one (Casale et al. 2008b) but needs to be better assessed. On the other hand, collection of a larger number of samples requires a facility with appropriate holding tanks and short-term husbandry capabilities.
The following recommendations can be derived. Feces collection should be routinely integrated with necropsies, to understand if and how the two methods differently represent prey consumption. Together, they provide a detailed assessment of prey consumption but offer only a short time range insight of the feeding behavior. For this reason, sampling should be carried out across all seasons, so that potential changes in habitat and distribution can be captured and eventually show differences in trophic position and/or behavior, depending on diet differences. Stable isotope studies should be implemented in the area as they can expand and integrate that knowledge providing insights on the contribution of fast-digested items on the diet, on longer dietary time frames and movement patterns. A better understanding of discard consumption is needed to foster more adequate management of fishing activities, and improved fishing practices should be identified to reduce discard. Moreover, since turtles ingest plastic in low quantities but high frequency, the potential sub-lethal effects should be investigated. Thus, clarifying trophic position and resources use provides a better understanding of habitat and distribution of the species and allows ultimately to highlight exposure to threats, steering conservation efforts.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Baldi G, Salvemini P, Attanasio AP, Mastrapasqua T, Pepe AM, Ceriani SA, Oliverio M, Casale P (2022) Voluntary fishing logbooks are essential for unveiling unsustainable bycatch levels and appropriate mitigating measures: the case of sea turtles in the Gulf of Manfredonia, Adriatic Sea. Aquatic Conserv Mar Freshw Ecosyst 32:741–752. https://doi.org/10.1002/aqc.3798
Baldi G, Furii G, Del Vecchio M, Salvemini P, Vallini C, Angelini V, Pari S, Lombardi Moraes K, Profico C, Olivieri V, Margaritoulis D, Rees AF, Çurri A, Hochscheid S, Freggi D, Lazar B, Luschi P, Casale P (2023) Behavioural plasticity in the use of a neritic foraging area by loggerhead sea turtles: insights from 37 years of capture–mark–recapture in the Adriatic Sea (Mediterranean Sea). ICES J Mar Sci 80:210–217. https://doi.org/10.1093/icesjms/fsac227
Benhardouze W, Aksissou M, Tiwari M (2021) Analysis of digestive tract contents from loggerhead sea turtles Caretta caretta (Linnaeus, 1758) stranded along the Northwest coast of Morocco. Cah Biol Mar 62:205–215. https://doi.org/10.21411/cbm.a.ea15a5c4
Bjorndal KA (1997) Foraging ecology and nutrition of sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles, vol I. CRC Press, Boca Raton, pp 199–231
Bjorndal KA, Bolten AB, Martins HR (2003) Estimates of survival probabilities for oceanic-stage loggerhead sea turtles (Caretta caretta) in the North Atlantic. Fish Bull 101:732–736
Blasi MF, Tomassini L, Gelippi M, Careddu G, Insacco G, Polunin NVC (2018) Assessing resource use patterns of Mediterranean loggerhead sea turtles Caretta caretta (Linnaeus, 1758) through stable isotope analysis. Eur Zool J 85:72–88. https://doi.org/10.1080/24750263.2018.1435742
Bolten AB (2003) Active swimmers–passive drifters: the oceanic juvenile stage of loggerheads in the Atlantic system. In: Bolten AB, Witherington BE (eds) Loggerhead sea turtles. Smithsonian Institution Press, Washington, pp 63–78
Bolten AB (1999) Techniques for measuring sea turtles. In: Eckert KAB KL, Abreu-Grobois FA, Donnelly M (ed) Research and management techniques for the conservation of sea turtles. IUCN/SSC Marine Turtle Specialist Group. p 110–114
Broderick AC, Coyne MS, Fuller WJ, Glen F, Godley BJ (2007) Fidelity and over-wintering of sea turtles. Proc R Soc B Biol Sci 274:1533–1539. https://doi.org/10.1098/rspb.2007.0211
Burke VJ, Standora EA, Morreale SJ (1993) Diet of Juvenile Kemp’s Ridley and Loggerhead Sea Turtles from Long Island, New York. Copeia 1993:1176–1180. https://doi.org/10.2307/1447107
Cardona L, Campos P, Levy Y, Demetropoulos A, Margaritoulis D (2010) Asynchrony between dietary and nutritional shifts during the ontogeny of green turtles (Chelonia mydas) in the Mediterranean. J Exp Mar Biol Ecol 393:83–89. https://doi.org/10.1016/j.jembe.2010.07.004
Casale P, Simone G (2017) Seasonal residency of loggerhead turtles Caretta caretta tracked from the Gulf of Manfredonia South Adriatic. Mediterr Mar Sci 18:4–10. https://doi.org/10.12681/mms.1663
Casale P, Freggi D, Basso R, Argano R (2004) Epibiotic barnacles and crabs as indicators of Caretta caretta distribution and movements in the Mediterranean Sea. J Mar Biol Assoc UK 84:1005–1006. https://doi.org/10.1017/S0025315404010318h
Casale P, Abbate G, Freggi D, Argano R (2008a) Caretta caretta (loggerhead sea turtle): digestion time. Herpetol Rev 39:343
Casale P, Abbate G, Freggi D, Conte N, Oliverio M, Argano R (2008b) Foraging ecology of loggerhead sea turtles Caretta caretta in the central Mediterranean Sea: evidence for a relaxed life history model. Mar Ecol-Prog Ser 372:265–276. https://doi.org/10.3354/meps07702
Casale P, Simone G, Conoscitore C, Conoscitore M, Salvemini P (2012) The Gulf of Manfredonia: a new neritic foraging area for loggerhead sea turtles in the Adriatic sea. Acta Herpetol 7:1–12. https://doi.org/10.13128/Acta_Herpetol-9897
Casale P, Broderick AC, Freggi D, Mencacci R, Fuller WJ, Godley BJ, Luschi P (2012b) Long-term residence of juvenile loggerhead turtles to foraging grounds: a potential conservation hotspot in the Mediterranean. Aquatic Conserv Mar Freshw Ecosyst 22:144–154. https://doi.org/10.1002/aqc.2222
Casale P, Freggi D, Paduano V, Oliverio M (2016) Biases and best approaches for assessing debris ingestion in sea turtles, with a case study in the Mediterranean. Mar Pollut Bull 110:238–249. https://doi.org/10.1016/j.marpolbul.2016.06.057
Casale P, Broderick AC, Camiñas JA, Cardona L, Carreras C, Demetropoulos A, Fuller WJ, Godley BJ, Hochscheid S, Kaska Y, Lazar B, Margaritoulis D, Panagopoulou A, Rees AF, Tomás J, Türkozan O (2018) Mediterranean sea turtles: current knowledge and priorities for conservation and research. Endanger Species Res 36:229–267. https://doi.org/10.3354/esr00901
Ceriani SA, Weishampel JF, Ehrhart LM, Mansfield KL, Wunder MB (2017) Foraging and recruitment hotspot dynamics for the largest Atlantic loggerhead turtle rookery. Sci Rep 7:16894. https://doi.org/10.1038/s41598-017-17206-3
De Leonardis C, Sandulli R, Vanaverbeke J, Vincx M, Zio S (2008) Meiofauna and nematode diversity in some Mediterranean subtidal areas of the Adriatic and Ionian Sea. Sci Mar 72:5–13. https://doi.org/10.3989/scimar.2008.72n15
Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44:842–852. https://doi.org/10.1016/S0025-326X(02)00220-5
Dodd CJ (1988) Synopsis of the biological data on the loggerhead sea turtle Caretta caretta (Linnaeus 1758). US Fish and Wildlife Service, Biological report 88. p 110
Epperly S, Braun McNeill J, Chester A, Cross F, Merriner J, Tester P (1995) Winter distribution of sea turtles in the vicinity of cape hatteras and their interactions with the summer flounder trawl fishery. Bull Mar Sci 56:547–568
Frick MG, Williams KL, Bolten AB, Bjorndal KA, Martins HR (2009) Foraging ecology of oceanic-stage loggerhead turtles Caretta caretta. Endanger Species Res 9:91–97. https://doi.org/10.3354/esr00227
Fukuoka T, Yamane M, Kinoshita C, Narazaki T, Marshall GJ, Abernathy KJ, Miyazaki N, Sato K (2016) The feeding habit of sea turtles influences their reaction to artificial marine debris. Sci Rep 6:28015. https://doi.org/10.1038/srep28015
Giannuzzi-Savelli R, Pusateri F, Palmeri A, Ebreo C (1999) Atlante delle conchiglie marine del Mediterraneo, vol 2. Evolver, Rome
Giannuzzi-Savelli R (1997) Atlante delle conchiglie marine del Mediterraneo: Caenogastropoda, parte 1: Discopoda-Heteropoda. Evolver
Handley SJ, Willis TJ, Cole RG, Bradley A, Cairney DJ, Brown SN, Carter ME (2014) The importance of benchmarking habitat structure and composition for understanding the extent of fishing impacts in soft sediment ecosystems. J Sea Res 86:58–68. https://doi.org/10.1016/j.seares.2013.11.005
Hatase H, Sato K, Yamaguchi M, Takahashi K, Tsukamoto K (2006) Individual variation in feeding habitat use by adult female green sea turtles (Chelonia mydas): are they obligately neritic herbivores? Oecologia 149:52–64. https://doi.org/10.1007/s00442-006-0431-2
Hatase H, Omuta K, Tsukamoto K (2007) Bottom or midwater: alternative foraging behaviours in adult female loggerhead sea turtles. J Zool 273:46–55. https://doi.org/10.1111/j.1469-7998.2007.00298.x
Haywood JC, Casale P, Freggi D, Fuller WJ, Godley BJ, Lazar B, Margaritoulis D, Rees AF, Shutler JD, Snape RT, Swain-Diaz NR, Widdicombe S, Broderick AC (2020) Foraging ecology of Mediterranean juvenile loggerhead turtles: insights from C and N stable isotope ratios. Mar Biol 167:28. https://doi.org/10.1007/s00227-020-3647-5
Hochscheid S, McMahon CR, Bradshaw CJA, Maffucci F, Bentivegna F, Hays GC (2007) Allometric scaling of lung volume and its consequences for marine turtle diving performance. Comp Biochem Physiol A Mol Integr Physiol 148:360–367. https://doi.org/10.1016/j.cbpa.2007.05.010
Hochscheid S, Travaglini A, Maffucci F, Hays GC, Bentivegna F (2013) Since turtles cannot talk: what beak movement sensors can tell us about the feeding ecology of neritic loggerhead turtles, Caretta caretta. Mar Ecol 34:321–333. https://doi.org/10.1111/maec.12018
Howell LN, Shaver DJ (2021) Foraging habits of Green sea turtles (Chelonia mydas) in the Northwestern Gulf of Mexico. Front Mar Sci 8:658368. https://doi.org/10.3389/fmars.2021.658368
Jones TT, Seminoff JA (2013) Feeding biology: advances from field-based observations, physiological studies, and molecular techniques. In: Wyneken J, Lohmann KJ, Musick JA (eds) The biology of sea turtles 3. CRC Press, Boca Raton, pp 211–247
Kaiser M, Collie JS, Hall SJ, Jennings S, Poiner I (2003) Impacts of fishing gear on marine benthic habitats. CABI Publishing, Wallingford, pp 197–217
Laurent L, Lescure J (1994) L’hivernage des tortues caouannes Caretta caretta (L.) dans le sud Tunisien. Revue D’ecologie Terre Et Vie 49:63–86
Lazar B, Gracan R (2011) Ingestion of marine debris by loggerhead sea turtles, Caretta caretta, in the Adriatic Sea. Mar Pollut Bull 62:43–47. https://doi.org/10.1016/j.marpolbul.2010.09.013
Lazar B, Gračan R, Katić J, Zavodnik D, Jaklin A, Tvrtković N (2011) Loggerhead sea turtles (Caretta caretta) as bioturbators in neritic habitats: an insight through the analysis of benthic molluscs in the diet. Mar Ecol 32:65–74. https://doi.org/10.1111/j.1439-0485.2010.00402.x
Limpus CJ, de Villiers DL, de Villiers MA, Limpus DJ, Read MA (2001) The loggerhead turtle, Caretta caretta in Queensland: feeding ecology in warm temperate waters. Mem Qld Mus 46:631–645
Lleonart J, Maynou F (2003) Fish stock assessments in the Mediterranean: state of the art. Sci Mar 67:37–49. https://doi.org/10.3989/scimar.2003.67s137
Mariani G, Bellucci F, Cocumelli C, Raso C, Hochscheid S, Roncari C, Nerone E, Recchi S, Di Giacinto F, Olivieri V, Pulsoni S, Matiddi M, Silvestri C, Ferri N, Renzo LD (2023) Dietary preferences of loggerhead sea turtles (Caretta caretta) in two mediterranean feeding grounds: does prey selection change with habitat use throughout their life cycle? Animals 13:654
Marini M, Campanelli A, Sanxhaku M, KljajiĆ Z, Grilli F (2015) Late spring characterization of different coastal areas of the Adriatic Sea. Acta Adriat 56:27–46
Martin J, Gambaiani D, Sabatte M-A, Pelorce J, Valentini A, Dejean T, Darmon G, Miaud C, Unmack P (2021) A comparison of visual observation and DNA metabarcoding to assess the diet of juvenile sea turtle. Mar Freshw Res 73:552–560. https://doi.org/10.1071/mf21179
Narazaki T, Sato K, Miyazaki N (2015) Summer migration to temperate foraging habitats and active winter diving of juvenile loggerhead turtles Caretta caretta in the western North Pacific. Mar Biol 162:1251–1263. https://doi.org/10.1007/s00227-015-2666-0
Nelms SE, Duncan EM, Broderick AC, Galloway TS, Godfrey MH, Hamann M, Lindeque PK, Godley BJ (2016) Plastic and marine turtles: a review and call for research. ICES J Mar Sci 73:165–181. https://doi.org/10.1093/icesjms/fsv165
OSTIA (2023) Global ocean OSTIA sea surface temperature and sea ice analysis
Palmer JL, Beton D, Çiçek BA, Davey S, Duncan EM, Fuller WJ, Godley BJ, Haywood JC, Hüseyinoğlu MF, Omeyer LCM, Schneider MJ, Snape RTE, Broderick AC (2021) Dietary analysis of two sympatric marine turtle species in the eastern Mediterranean. Mar Biol 168:94. https://doi.org/10.1007/s00227-021-03895-y
Plotkin PT, Wicksten MK, Amos AF (1993) Feeding ecology of the loggerhead sea turtle Caretta caretta in the Northwestern Gulf of Mexico. Mar Biol 115:1–5. https://doi.org/10.1007/bf00349379
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramirez MD, Avens L, Seminoff JA, Goshe LR, Heppell SS (2017) Growth dynamics of juvenile loggerhead sea turtles undergoing an ontogenetic habitat shift. Oecologia 183:1087–1099. https://doi.org/10.1007/s00442-017-3832-5
Revelles M, Cardona L, Aguilar A, Fernández G (2007) The diet of pelagic loggerhead sea turtles (Caretta caretta) off the Balearic archipelago (western Mediterranean): relevance of long-line baits. J Mar Biol Assoc UK 87:805–813. https://doi.org/10.1017/s0025315407054707
Riedl R (1991) Fauna e flora del Mediterraneo. Franco Muzzio Editore, London, p 777
Roast MJ, Martins S, Fernández-Peralta L, Báez JC, Diame A, March D, Ouled-Cheikh J, Marco A, González-Solís J, Cardona L (2023) Hidden demographic impacts of fishing and environmental drivers of fecundity in a sea turtle population. Conserv Biol. https://doi.org/10.1111/cobi.14110
Sabelli B, Minelli A (2009) Atlante di diversità e morfologia degli invertebrati. Piccin, Padua
Sánchez P, Recasens L, Demestre M, Sartor P, Ligas A, Martín J, Ranieri Sd (2007) Trawl catch composition during different fishing intensity periods in two Mediterranean demersal fishing grounds. Sci Mar 71:765–773
Schuyler Q, Hardesty BD, Wilcox C, Townsend K (2014) Global analysis of anthropogenic debris ingestion by sea turtles. Conserv Biol 28:129–139. https://doi.org/10.1111/cobi.12126
Seney EE, Musick JA (2007) Historical diet analysis of loggerhead sea turtles Caretta Caretta in Virginia. Copeia 2007:478–489. https://doi.org/10.1643/0045-8511(2007)7[478:HDAOLS]2.0.CO;2
Senko JF, Nelms SE, Reavis JL, Witherington B, Godley BJ, Wallace BP (2020) Understanding individual and population-level effects of plastic pollution on marine megafauna. Endanger Species Res 43:234–252. https://doi.org/10.3354/esr01064
Simpson EH (1949) Measurement of diversity. Nature 163:688. https://doi.org/10.1038/163688a0
Sokal RR, Sneath HA (1963) Princiales of numerical taxonomy. WH Freeman & Co., San Francisco and London
Solomando A, Pujol F, Sureda A, Pinya S (2022) Ingestion and characterization of plastic debris by loggerhead sea turtle, Caretta caretta, in the Balearic Islands. Sci Total Environ 826:154159. https://doi.org/10.1016/j.scitotenv.2022.154159
Storelli MM, Storelli A, Marcotrigiano GO (2001) Heavy metals in the aquatic environment of the Southern Adriatic Sea, Italy: macroalgae, sediments and benthic species. Environ Int 26:505–509. https://doi.org/10.1016/S0160-4120(01)00034-4
Tolve L, Casale P, Formia A, Garofalo L, Lazar B, Natali C, Novelletto A, Vallini C, Bužan E, Chelazzi G, Gaspari S, Fortuna C, Kocijan I, Marchiori E, Novarini N, Poppi L, Salvemini P, Ciofi C (2018) A comprehensive mitochondrial DNA mixed-stock analysis clarifies the composition of loggerhead turtle aggregates in the Adriatic Sea. Mar Biol 165:1–14. https://doi.org/10.1007/s00227-018-3325-z
Tomas J, Aznar FJ, Raga JA (2001) Feeding ecology of the loggerhead turtle Caretta caretta in the western Mediterranean. J Zool 255:525–532. https://doi.org/10.1017/s0952836901001613
Tomas J, Guitart R, Mateo R, Raga JA (2002) Marine debris ingestion in loggerhead sea turtles, Caretta caretta, from the Western Mediterranean. Mar Pollut Bull 44:211–216. https://doi.org/10.1016/s0025-326x(01)00236-3
Tsagarakis K, Palialexis A, Vassilopoulou V (2014) Mediterranean fishery discards: review of the existing knowledge. ICES J Mar Sci 71:1219–1234. https://doi.org/10.1093/icesjms/fst074
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Wallace BP, Avens L, Braun-McNeill J, McClellan CM (2009) The diet composition of immature loggerheads: Insights on trophic niche, growth rates, and fisheries interactions. J Exp Mar Biol Ecol 373:50–57. https://doi.org/10.1016/j.jembe.2009.03.006
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B 73:3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x
Acknowledgements
We thank Prof. A Castelli who helped with identification of Ctenophora, and all the volunteers who helped with handling animals at the rescue center of Associazione Panda Molfetta. We also thank two anonymous reviewers for their valuable comments on a first version of the manuscript.
Funding
Open access funding provided by Università di Pisa within the CRUI-CARE Agreement. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: PC; methodology: PC, GB; formal analysis: GB, MM; investigation: MM, PS; resources: PS, PC; data curation: MM; writing—original draft: GB, MM; writing—review and editing: PC, PS; visualization: GB; supervision: PC; project administration: PS, PC.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no conflicts of interest to declare. PC is an Associate Editor of the Journal.
Ethics approval
The study did not involve human participants. Animals were handled within a research project authorized by the Italian Ministry of Environment (Ministero dell’Ambiente e della Tutela del Territorio e del Mare, prot. 0000863 16/01/2019).
Additional information
Responsible Editor: L. Avens.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baldi, G., Miglianti, M., Salvemini, P. et al. Diet of loggerhead turtles in the Gulf of Manfredonia, South Adriatic Sea: evidence of winter feeding and anthropogenic impacts. Mar Biol 170, 169 (2023). https://doi.org/10.1007/s00227-023-04316-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04316-y