Abstract
Two new pygmy squid from the Ryukyu archipelago, Japan, are described: Kodama jujutsu, n. gen., n. sp. and Idiosepius kijimuna, n. sp. They differ from all other nominal species in a combination of traits, including the number of tentacular club suckers, shape of the funnel-mantle locking-cartilage, modification of the male hectocotylus and the structure of the gladius and nuchal-locking cartilage, in addition to mitochondrial DNA markers (12S, 16S and COI). They are both known from Okinawa Island and there is some overlap in their distributions. In a molecular phylogeny that includes all nominal Idiosepiidae, Kodama jujutsu, n. gen., n. sp. is sister taxon to a clade containing Xipholeptos Reid & Strugnell, 2018 and Idiosepius Steenstrup, 1881. Xipholeptos and Idiosepius are sister taxa. Idiosepius spp. now includes seven nominal species. In addition, aspects of the behaviour of the new species are described.
Similar content being viewed by others
Introduction
In a recent study of Idiosepius Steenstrup, 1881 an undescribed species was recognised from Okinawa, Japan based on morphological and molecular traits (Reid and Strugnell 2018). It was represented by two males and a single female specimen held in the Australian Museum collections. The focus in Reid and Strugnell (2018) was on the Australian representatives of the family and because only two preserved specimens were available for examination at the time, the species was not formally described and was recognised as ‘Okinawa’ n. sp. (Reid and Strugnell 2018: 472). Since then, more specimens have been collected enabling the species to be fully described here. It has been found at a number of locations in the Ryukyu Island archipelago, ranging from off Hamamoto, Okinawa Island in the north, and south to Sakiyama Bay, Iriomote Island.
Subsequently, a second idiosepiid was found in the region that did not appear to conform to other known idiosepiids. It shares some morphological traits with the southern Australian endemic Xipholeptos notoides (Berry, 1921). Both of the Japanese taxa were included in a molecular analysis with representatives of all known Idiosepiidae. The taxa within this family have historically proved difficult to identify based only on morphology (von Byern and Klepal 2010) but the application of molecular tools is facilitating a much better understanding of species boundaries and uncovering some hitherto interesting systematic depth within the family (von Byern et al. 2012; Reid and Strugnell 2018).
Both taxa are described below with some behavioural observations based on wild and laboratory-reared animals. Live animal videos of both species are included in the Supplementary information. These observations are compared with what is currently known about other members of the family.
Material and methods
Morphology
Terminology, measurements, indices, and abbreviations for anatomical structures follow Reid and Strugnell 2018 (based on Roper and Voss 1983) and are listed in Table 1. All measurements are in millimetres (mm). Measurements and counts for individual mature specimens of the new species are presented in Tables 3, 4, 6 and 7; the range of values for each character is expressed in the descriptions and in Tables 2 and 5 as: minimum–mean–maximum (SD). The values for each sex are given separately. In the case of discrete probability distributions, such as sucker-counts, standard deviations are not provided in cases where counts are few in number because these cannot be calculated using the same formula as can be applied to normally distributed data.
For scanning electron microscopy, arms, clubs and radulae were removed, mounted, then air dried and examined in a Zeiss Evo LS15 SEM using a Robinson Backscatter detector. Soft structures were mounted directly on carbon tape and air dried; some radulae and gladii were mounted on glass coverslips prior to SEM. Photomicrography using a compound microscope was also used to illustrate some structures.
Other abbreviations
AMS, Australian Museum, Sydney; NSMT, National Museum of Nature and Science, Tokyo, Japan; WAM, Western Australian Museum, Perth.
Taxon sampling and DNA isolation
Specimens were collected at night by wading, snorkelling, or on SCUBA from shore at Hamamoto (26° 40′ 17.90″ N, 127° 53′ 17.05″ E), Sunabe (26° 19′ 41.09′′ N, 127° 44′ 35.98′′ E), Maeda (26° 26′ 43.54′′ N, 127° 46′ 20.01′′ E), Onna Point (26° 40′ 17.90.4′′ N, 127° 50′ 28.78′′ E), Miyagi (26° 22′ N, 127° 59′ E), and Kaichyu-doro (26° 19′ N, 127° 55′ E) near Okinawa Island and Sakiyama Bay (24° 19′ N, 123° 40′ E) and Shirahama (24° 22′ N, 123° 44′ E) near Iriomote Island. Adults of both species were collected using dipnets or 200 ml transparent plastic containers. After collection they were transferred to 20 L buckets of fresh seawater and aerated with a battery powered bubbler and immediately transported to the Okinawa Institute of Science and Technology where they were gradually acclimated over one hour to 20 °C and other aquarium parameters and conditions used by Jolly et al. (2022). Adults were kept in a 70 L tank where they were observed until sacrificed. They were first transferred to a 500 ml container of filtered seawater and anaesthetised in a magnesium chloride solution (Abbo et al. 2021). Magnesium chloride was gradually added to a final concentration of 7% over 30 min. After no response to pinching stimuli and total cessation of breathing was observed, a small fin clip biopsy was performed for DNA extraction. The animal was then transferred to 4% methanol free paraformaldehyde in seawater and fixed for 72 h at 4 °C with gentle rocking. Specimens were then transferred to 70% ethanol for long-term preservation. Some specimens were fixed and preserved in 95% ethanol.
Freshly collected specimens were used for molecular study and analyses. Previously sequenced individuals were used to increase sample size with specimen data obtained from GenBank. Taxon names applied were those assigned by Reid and Strugnell (2018) and include: Xipholeptos notoides (Berry, 1921); I. hallami Reid and Strugnell 2018; I. minimus d’Orbigny in Férrusac and d’Orbigny 1835; I. paradoxus (Ortmann, 1888); I. picteti (Joubin, 1894); I. pygmaeus Steenstrup, 1881, and I. thailandicus Chotiyaputta et al, 1991. As used in that analysis, Semirossia patagonica (Smith, 1881) was selected as the outgroup taxon because among available mtDNA genomes, the Idiosepius sequence shows greatest similarity to that of Semirossia (Hall et al. 2014) and in addition, a sister taxon relationship between Idiosepiidae and Sepiolida is supported in the literature (Strugnell et al. 2017). Tissue from 16 new individuals that included both suspected new species was sampled (See Appendix Table 9). DNA was extracted using commercial kits (NucleoSpin; Machery-Nagel, Germany or DNeasy Blood & Tissue Kit; Qiagen, Hilden, Germany) according to the manufacturer’s protocols.
PCR amplification and nucleotide sequencing
Partial sequences of three mitochondrial genes; 12S rRNA, 16S rRNA and cytochrome c oxidase subunit I (COI) were amplified in this study. Primers and annealing temperatures are detailed in Allcock et al. (2008). PCR was performed in a 20 µL volume, containing 2.0 µL Ex Taq buffer, 1.6 µL of dNTP, 0.1 µL of TaKaRa Ex Taq DNA Polymerase (TaKaRa), 1.0 µL of each primer, 12.3 µL of distilled H2O and 2.0 µL template DNA. PCR products were sequenced by a commercial sequencing service (Fasmac, Japan) in both directions.
Molecular sequence analyses
DNA sequences were compiled in Geneious v 9.0.5 and sequences were aligned using MAFFT v7.222 (Katoh and Kuma 2002). PartitionFinder v1.1.1 (Lanfear et al. 2012) was used to select best-fit partitioning schemes and evolutionary models for the genes contained within the alignment. Maximum likelihood phylogenies were estimated for all datasets using PhyML (Guindon and Gascuel 2003), IQ-TREE (Nguyen et al. 2015) implemented within the W-IQ-TREE web interface (Trifinopoulos et al. 2016) and also RAxML (Stamatakis 2014) implemented within the RAxML BlackBox web server (Stamatakis et al. 2008).
Results
Phylogenetic analysis
The best fit model was one where all three genes were contained within a single partition. The evolutionary model with the lowest BIC values was the Hasegawa-Kishino-Yano Model (HKY) + I + G (Hasegawa et al. 1985). The total evidence tree obtained from the sequence data is shown in Fig. 1.
Maximum Likelihood phylogenetic tree generated using PhYML (GTR + I + G) from the analysis of partial fragments of 12S rRNA, 16S rRNA and CO1. Semirossia patagonica was used the outgroup. Bootstrap values (1000 replicates) were generated from maximum likelihood analysis using IQ-tree/RAMxML/PhyML. Taxon names to the left of the shaded bar refer to the species names used in GenBank records and associated publications, in addition to the new taxa identified in this analysis. Taxon names to the right of the bar are those we believe should be assigned to the studied taxa. Numbers to the right of the taxon names refer to sample numbers corresponding to individual specimens sequenced that are listed in the Appendix Table 9; numbers in square brackets were sequenced for this study, those without brackets correspond to specimens included in Reid and Strugnell (2018)
A number of well-supported clades were retrieved from the analysis, with clear structuring within some of the larger clades. Two clades that include the newly sequenced taxa from the Ryukyu archipelago, are clearly distinct and well-supported. Xipholeptos notoides (IQ-tree bootstrap [IQ-BS] = 100%; RAxML bootstrap [R-BS], R-BS = 100%, PhyML bootstrap P-BS = 100%) was sister-taxon to a clade containing all Idiosepius (IQ-tree bootstrap [IQ-BS] = 98%; RAxML bootstrap [R-BS], R-BS = 77%, PhyML bootstrap P-BS = 93%). As reported in Reid and Strugnell (2018), the branch length separating X. notoides from Idiosepius is long relative to the branch lengths within Idiosepius indicating considerable molecular divergence.
Also on a long branch is a clade that includes one of the newly discovered Okinawan idiosepiids (IQ-tree bootstrap [IQ-BS] = 100%; RAxML bootstrap [R-BS], R-BS = 100%, PhyML bootstrap P-BS = 100%). This taxon is sister to the southern Australian endemic X. notoides and Idiosepius spp. Together with some significant morphological traits, deep evolutionary divergence is suggested based on analysis of the molecular data. For these reasons we place the members of this clade in its own genus, Kodama n. gen. as described below.
The second Japanese taxon forms a well-supported clade within Idiosepius (IQ-tree bootstrap [IQ-BS] = 100%; RAxML bootstrap [R-BS], R-BS = 91%, PhyML bootstrap P-BS = 99%). Members of this clade are recognised and described below as a new species, Idiosepius kijimuna n. sp. It includes the taxa referred to as ‘Okinawa’ n. sp. in Reid and Strugnell (2018) and is sister to Idiosepius minimus. This clade shows some considerable internal structure, particularly among three termini that have a high support value on a relatively long branch (IQ-tree bootstrap [IQ-BS] = 92%; RAxML bootstrap [R-BS], R-BS = 82%, PhyML bootstrap P-BS = 84%).
Systematic descriptions
Idiosepius kijimuna n. sp.
(Figs. 1, 2, 3, 4, 5, 6, Tables 2, 3, 4, 8, Appendix Table 9).
Idiosepius kijimuna n. sp. a dorsal view, male, 5.4 mm ML, AMS C.575592. b ventral view, same specimen. c dorsal view, female, 11.2 mm ML, AMS C.477896. d ventral view of head showing ventro-lateral skin tags (arrows), male, 5.4 mm ML, AMS C.575592. e funnel, paratype female, 8.6 mm ML, NSMT Mo-85875. f funnel-locking cartilage, paratype male, 7.2 mm ML, NSMT-Mo 85881. g mantle-locking cartilage, male as in f. Scale bars: a, b = 1 mm; c = 2 mm; d = 0.5 mm; e = 1 mm; f, g = 200 µm
Idiosepius kijimuna n. sp. a funnel organ stained with methylene blue, paratype male, 7.2 mm ML, NSMT-Mo 85881. b nuchal cartilage, specimen as in a. c SEM ventral view of portion of arm crown (arms 1–3, numbered), paratype male, 6.0 mm ML, NSMT-Mo 85878. d SEM enlargement of arm 2 (left) and arm 3 (right) suckers, specimen as in c. e enlargement of individual sucker rim, arm 3 left, specimen as in c. f ventral arms, male 5.4 mm ML (AMS C.575592). g SEM hectocotylised left arm 4, paratype male 6.0 mm ML, NSMT-Mo 85878. h far left, hectocotylised left ventral arm (tip recurved during fixation), male AMS C.575592, 5.4 mm ML. Scale bars: a, b, = 200 µm; c = 300 µm, d = 30 µm, e = 3 µm, f = 0.5 mm. g, h = 200 µm
Idiosepius kijimuna n. sp. a SEM, tentacular club sucker, male paratype, 6.0 mm ML, NSMT-Mo 85858. b radula, female, 10.2 mm ML, AMS C.477896. c enlargement of portion of radula. d upper beak, specimen as in b. e lower beak, lateral view, specimen as in d. f enlargement of oral end of spermatophore, male, 6.5 mm ML, NSMT-Mo 85924. Scale bars: a = 10 μm; b, c = 1 mm; d, e = 2 mm; f = 1 mm
Common name: Ryukyu Pygmy Squid; Japanese name, Ryukyu-himeika.
Material examined
Type material
Holotype: Japan, Okinawa I., Motobu Hamamoto [Peninsula], 26° 40′ N, 127° 53′ E, coll. J. Jolly: 1♂, 8.1 mm ML, 22 Feb. 2019 (NSMT-Mo 85928).
Paratypes: Japan, Okinawa I., Motobu Hamamoto [Peninsula], 26° 40′ N, 127° 53′ E, coll. N. Sato: 1♀, 8.6 mm ML, 26 Jan. 2007 (NSMT-Mo 85875); 1♀, 10.0 mm ML, 19 Apr. 2007 (NSMT-Mo 85876); 1♂, 6.6 mm ML, 1♀, 7.2 mm ML, 3 May 2007 (NSMT-Mo 85877); 3♂, 3.8–6.5 mm ML, 4 May 2007 (NSMT-Mo 85878); 4♂, 4.0–6.2 mm ML, 1♀, 5.6 mm ML, 15 May 2007 (NSMT-Mo 85879); 5♂, 4.0–5.7 mm ML, 1♀, 7.3 mm ML, 18 May 2007 (NSMT-Mo 85880); 1♀, 7.4 mm ML, 29 Aug. 2007 (NSMT-Mo 85881); 1♂, 5.2 mm ML, 29 Oct. 2007 (NSMT-Mo 85882).
Other material examined
Japan: 4♀, 4.0–5.2 mm ML, Iriomote I., Sakiyama Bay, 24° 19′ N, 123° 40′ E, coll. N. Sato, 26 Aug. 2014 (AMS C.596044); 7♂, 6♀, Iriomote I., Shirahama, 24° 22′ N, 123° 44′ E, coll. N. Sato, 27 Aug. 2014 (AMS C.596045). Okinawa I.: Kaichyu-doro, 26° 19′ N, 127° 55′ E, coll. N. Sato: 1♂, 4.3 mm ML, 25 Jun. 2007 (AMS C.596040); 1♀, 9.4 mm ML, 3 Jul. 2007 (AMS C.596041). Miyagi I., 26° 22′ N, 127° 59′ E, coll. N. Sato: 1♂, 6.1 mm ML, 1♀, 10.0 mm ML, 23 Jan. 2008 (NSMT-Mo 85884); 1♂, 6.5 mm ML, 21 Feb. 2008 (AMS C.596042), 1♂, 5.2 mm ML, 3 Jun. 2008 (AMS C.596043). Motobu Hamamoto [Peninsula], 26° 40′ N, 127° 53′ E, coll. N. Sato, 9 Dec. 2007 (NSMT-Mo 85883); 2♂, 7.3 mm ML, 5.3 mm ML, data as for previous specimen, 22 Mar. 2007 (AMS C.596039). Okinawa, Hamamoto, 26° 40′ N, 127° 53′ E, coll. J. Jolly, 22 Feb. 2019: 1♂, 6.2 mm ML (NSMT-Mo 85926); 1♂, 7.4 mm ML, 1♀, 6.9 mm ML (NSMT-Mo 85923); 1♂, 6.5 mm ML, 1♀, 6.2 mm ML (NSMT-Mo 85924); 1♀, 10.0 mm ML (NSMT-Mo 85921); 1♀, 11.4 mm ML (NSMT-Mo 85922); 1♀, 9.8 mm ML (NSMT-Mo 85925); 1♀, 11.8 mm ML (NSMT-Mo 85927); 1♀, 7.8 mm ML (NSMT-Mo 85929); 1♀, 11.5 mm ML (NSMT-Mo 85930); 1♀, 11.0 mm ML (NSMT-Mo 85931); 1♀, 6.8 mm ML (AMS C.596046); 1♀, 6.8 mm ML (AMS C.596047); 1♀, 7.0 mm ML (AMS C.596048); 1♀, 9.3 mm ML (AMS C.596049); 1♀, 6.0 mm ML (AMS C.596050); 1♀, 6.5 mm ML (AMS C.596051); 1♀, 8.5 mm ML (AMS C.596052); 1♀, 7.0 mm ML (AMS C.596053); 1♀, 7.8 mm ML (AMS C.596054); 3 juv., 1.9–2.0 mm ML (AMS C.596055). Motobu Pen. Bise, 26° 42′ 31″ N, 127° 52′ 42″ E, 26 May 2013, coll. H. Fukumori, K. Hidaka & Y. Takano: 1♀, 11.2 mm ML (AMS C.477896); 1♂, 5.4 mm ML (AMS C.575592); 1♂, 4.7 mm ML (AMS C.575591).
Diagnosis
Tentacular club with two suckers in each transverse row; total number of club suckers 32–40♂, 42–50♀. Male hectocotylised arms 4 longer than remaining arms; right ventral arm longer than left ventral arm (Fig. 2a, b; Table 2). Female arms 1 shorter than remaining arms, rest similar in length. Hectocotylus: male left ventral arm with 3–6 suckers basally and large flap at tip of arm; right ventral arm with 2–4 suckers basally. GiLC males 14–18; females 20–25.
Description
Counts and indices for individual specimens are given in Tables 3 (males) and 4 (females). Ten mature male and ten mature females were measured.
Mature males smaller than females: ML males 5.4–6.4–8.1 mm (SD 0.8), females 9.8–10.8–11.8 mm (SD 0.7). Mantle blunt-cylindrical (Fig. 2a–c); MWI males 46.2–59.3–70.2 mm (SD 7.8), females 52.0–57.8–63.3 mm (SD 3.5). Dorsal mantle not joined to head, ventral mantle margin straight to slightly concave. Ventral skin tags present, one on each side of head posterior to eyes (Fig. 2d). Fins small, rounded, length approximately one-third mantle length, FLI males 31.1–36.6–42.1 mm (SD 3.3), females 27.3–33.4–38.0 mm (SD 3.3); positioned dorso-laterally on posterior end of mantle, FIIa males 64.5–74.3–80.0 mm (SD 4.3), females 66.4–71.7–78.6 mm (SD 4.1); fin width ~ 20% ML, FWI males 19.8–24.5–29.6 mm (SD 3.2), females 13.6–18.2–23.2 mm (SD 3.0); anterior and posterior margins with well-developed lobes, lateral lobes crescentic.
Funnel conical, base broad, tapered anteriorly (Fig. 2e); FuLI males 21.5–26.6–32.4 mm (SD 3.4), females 20.2–24.6–30.0 mm (SD 3.3); free for about 1/3 of its length, FFuI males 12.3–15.9–21.5 mm (SD 3.4), females 20.2–24.6–30.0 mm (SD 3.3). Funnel-locking cartilage (Fig. 2f), deep, oval, with defined outer rim. Mantle-locking cartilage (Fig. 2g) compliments funnel member, an ear-shaped lug, broadest posteriorly, tapering towards mantle margin. Funnel valve small, flaplike, rounded anteriorly, dorsal element broad, inverted V-shape with pointed anterior tip; ventral elements ovoid (Fig. 3a). Nuchal locking cartilage oval, not well-defined, indistinct (Fig. 3b).
Head broader than long in both sexes, HLI males 36.3–44.6–51.5 mm (SD 4.9), females 28.2–32.5–38.2 mm (SD 3.4); HWI males 39.5–52.0–66.7 mm (SD 8.7), females 36.4–40.8–47.0 mm (SD 3.2). Eyes large, EDI males 8.1–10.2–14.8 mm (SD 2.4), females 6.4–8.3–11.7 mm (SD 1.4); ventral eyelids free. Eye covered by corneal membrane. Distinct, large olfactory pit on latero-posterior surface of head, posterior and ventral to eyes, close to mantle opening.
Arms, broad basally, tapered distally, hectocotylised arms much longer than unmodified arms in males 4.3.2.1 or 4.2.3.1 (Tables 2 and 3), arm formula variable in females, with arm 4 usually longest and arms 1 shorter than lateral arms (Tables 2 and 4). Arm length index of longest arm in males (ALI4 right) 37.0–48.2–61.4 mm (SD 7.2), females (ALI4) 22.0–26.7–31.8 mm (SD 3.0). All arms similar in shape, U-shaped in section (Fig. 3c). Sucker pedicels broad, short, suckers joined closely to arms and club. Chitinous inner ring of arm suckers without teeth, smooth or slightly crenulated on inner margin (Fig. 3d). Infundibulum with 2–3 rows of shallow cup-like broad-based pegs, innermost row of pegs larger, slightly more elongate, cylindrical surrounding inner ring, more elongate on one side of the sucker than the other; processes contain low papillae, outer-most sucker rim processes rectangular, flat, radially arranged (Fig. 3d, e). Male and female arm suckers similar in size (Table 2). Sucker counts range from 13–24 on male normal arms, 20–32 in females. All arms connected by relatively shallow webs, protective membranes absent.
Both ventral arms of males hectocotylised: (Fig. 3f–h). Right ventral arm with 2.0–3.0–4.0 suckers proximally remainder of arm without suckers; aboral side of arm with broad, thin, ventro-lateral flanges attached laterally on each side of arm; flange broadest proximally, tapering to distal tip of arm (Fig. 3f). Right ventral arm slightly longer than left ventral arm (Table 2). Left ventral arm with 3.0–5.0–6.0 suckers proximally (generally a greater number of suckers on left than on right ventral arm), remainder of arm without suckers; distal tip of arm flattened forming a blunt tongue-like flange and proximal to this a slightly shorter blunt flap; in preserved specimens, distal flap often recurved to cover distal tip of arm (Fig. 3g, h).
Tentacles similar to arms in appearance, semicircular in section; oral surface convex. Club relatively long; ClLI males 27.2–32.8–42.6 mm (SD 4.8), females 32.0–34.9–39.5 mm (SD 2.2), cylindrical, tapers to blunt end distally. Sucker-bearing face of club only slightly convex. Suckers ~ 0.1–0.2 mm diameter in centre of club, arranged in two rows in both sexes. Total number of club suckers in males 32.0–35.0–40.0 (SD 3); females 42.0–47.2–51.0. Swimming keel on aboral side of carpus broad, extends posteriorly beyond carpus. Keel forms groove on oral side. Club sucker dentition (Fig. 4a): inner ring without teeth; infundibulum with 3 rows of pegs; shallow, cup-like distally bearing numerous papillae. At periphery, pegs narrower and with fewer papillae. Outer-most sucker rim processes flattened, rectangular.
Gills with 14–16–18 (SD 1.6) lamellae per demibranch in males, females with 20–22–25 (SD 2.0) lamellae per demibranch; GiLI 17.9–24.7–28.4 mm (SD 2.9) males, 18.8–25.8–30.6 mm (SD 4.2) females.
Buccal membrane with six lappets and fringed inner margin; suckers absent. Radula with seven transverse rows of homodont teeth (Fig. 4b). Rachidian teeth, and second lateral teeth broad basally, tapering distally, second laterals asymmetrical with cusp of displaced toward midline of radula ribbon (Fig. 4b, c), second lateral teeth triangular, pointed, symmetrical in shape; marginal teeth narrow, scythe-like (Fig. 4b).
Upper beak (Fig. 4d) with short, triangular rostrum, and, as for lower beak, flanked by row of smaller teeth. Lower beak (Fig. 4e) with large median rostrum, flanked on either side with a row of similar-sized small teeth. Distinct dark pigmentation restricted to rostrum of upper and lower beaks.
Male reproductive tract similar in structure to congeners (not illustrated). Spermatophores approximately 1/3 mantle length; SpLI 28.7–31.2–33.3 mm (SD 2.9), SpWI 1.9–2.1–2.4 mm (SD 0.2). Sperm reservoir simple, without coiled sperm cord. Cement body unipartite; aboral end cup-shaped, cylindrical, oral end tapering toward ejaculatory apparatus (Fig. 4f). Oral end of ejaculatory apparatus with 3–4 simple coils.
Female reproductive tract: Ovary large, occupies approximately half of mantle cavity. Eggs of various sizes suggesting protracted multiple spawning. Ovary opens via single thick-walled oviduct at anterior end on left side of animal. Nidamental glands paired, broad, leaf-shaped, located ventral to ovary toward, and overlying anterior half. Accessory nidamental glands absent. Eggs ovoid, 0.8–1.3 mm diameter; EgDI 7.1–9.3–12.6 mm (SD 1.4).
Gladius reduced to a thin, ovoid, chitinous structure embedded in ventral side of dorsal mantle below adhesive pad; does not extend full length of mantle. Rachis absent.
Preserved animals cream with purple chromatophores evenly peppered dorsally and ventrally on mantle and arms, largest on head (Fig. 2a–c). Large chromatophores on arms appear as block-like bands. Chromatophores on fins confined to junction with mantle, do not extend to outer fin margins. Ventral side of funnel with chromatophores. Chromatophores often in a row at distal tip. Chromatophores in tissue overlying internal viscera. Live animals (Fig. 5) mid-brown to greenish brown with relaxed chromatophores giving a predominantly uniform colouration (Fig. 5a).
Type locality
Japan, Okinawa I., Motobu Hamamoto [Peninsula], 26° 40′ N, 127° 53′ E.
Distribution
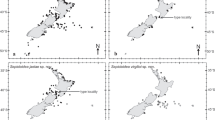
Japan: Ryukyu Islands: Iriomote I., Sakiyama Bay, 24° 19′ N, 123° 40′ E to Okinawa, Motobu Pen. Bise, 26° 42′ 31″ N, 127° 52′ 42″ E (Fig. 6).
Habitat and biology
Idiosepius kijimuna have primarily been collected from shallow (less than 2 m) seagrass beds in Okinawa in winter from November to March. During this time, they have also been observed, albeit rarely, in coral habitats. Their whereabouts during the warmer months are largely unknown.
Etymology
The species name is used for creatures in Okinawan mythology. The Kijimunā are said to be elfin creatures that make their home in the banyan trees that grow over the Ryukyu Archipelago. Their diet consists entirely of seafood and they are excellent fishers. They avoid octopuses at all costs. The name is used as a noun in apposition.
Remarks
Characters that distinguish I. kijimuna from Kodama jujutsu, n. sp. n. gen. and X. notoides are provided in the Diagnosis and Remarks under K. jujutsu n. sp. n. gen. below. The combination of characters that separate I. kijimuna from other nominal Idiosepius are provided in Table 8.
The presence of relatively long male ventral arms is not unique to I. kijimuna. This trait is shared by its sister taxon I. minimus and also I. thailandicus. These three taxa together form a well supported clade within the broader Idiosepius clade (IQ-tree bootstrap [IQ-BS] = 97%; RAxML bootstrap [R-BS], R-BS = 81%, PhyML bootstrap P-BS = 79%) that appears to be sister to I. picteti (Fig. 1).
Kodama n. gen.
Type species
Here designated. Kodama jujutsu, n. sp.
Diagnosis
Mantle-locking cartilage a straight ridge, funnel-locking cartilage a corresponding straight, narrow furrow. Eight to 10 pairs of suckers, extend along length of both ventral arms in males; aboral side of right ventral arm not modified, smooth; only left ventral arm modified as the hectocotylus. Tentacular club with two rows of suckers in transverse rows. Gills with 15–18 lamellae per demibranch. Rachidian teeth of radula homodont; first lateral teeth short, much smaller than other teeth and cusps low. Gladius extends full length of mantle, paddle-shaped, narrow, pointed anteriorly, broadens midway along its length; remainder of gladius, clear non-sclerotised. Nuchal-locking cartilage distinct, well-defined. Prominent skin tags, one on each side, posterior to eyes; anterior to eyes on ventral side of head with pair of prominent, small rounded, protruding papillae.
Etymology
The generic name Kodama refers to a tree spirit in Japanese folklore. It has a reputation of being rounded in shape. The presence of Kodama is a sign of a healthy forest. We have chosen this name to suggest its extension to representing a healthy reef.
Remarks
Some traits of Kodama n. gen. are shared with Xipholeptos. Both of these monotypic taxa have a straight mantle-locking cartilage, and the funnel-locking cartilage is a corresponding straight furrow. Both have a medial rachis in the gladius and the arms of males are all similar in length. The aboral side of the right ventral arm is without a keel, and posterior to the eyes is a pronounced skin tag. These traits separate Kodama n. gen. and Xipholeptos from Idiosepius. However, the distinct, folded bipartite structure seen in the aboral end of the spermatophore cement body reported for X. notoides in Reid and Strugnell (2018) is not present in Kodama n. gen. In addition, the club has two transverse rows of suckers in Kodama, n. gen. and four rows in Xipholeptos. The body of Xipholeptos is cylindrical and elongate, while that of Kodama is squat and rounded.
Kodama jujutsu, n. sp.
(Figs. 1, 6, 7, 8, 9, 10, 11, 12, Tables 5, 6, 7, 8, Appendix Table 9).
Kodama jujutsu n. gen., n. sp. a dorsal view, male paratype, 5.3 mm ML, NSMT-Mo 85933. b ventral view, specimen as in a. c funnel, ventral view, specimen as in b. d funnel-locking cartilage, paratype female, 12.9 mm ML, NSMT-Mo 85937. e mantle-locking cartilage, specimen as in d. f, funnel organ stained with methylene blue, male paratype, 5.3 mm ML, NSMT-Mo 85933. g nuchal-locking cartilage, female paratype 8.7 mm ML. Scale bars: a, b = 1 mm, c = 1 mm, d, e = 0.5 mm, f = 200 µm, g = 0.5 mm
Kodama jujutsu n. gen., n. sp. a radula, male, unregistered specimen collected 7 Dec 2019. b upper beak, male paratype, 6.0 mm ML, NSMT-Mo 85940. c lower beak, specimen as in b. d spermatophore, male paratype 5.3 mm ML, NSMT-Mo 85933. e enlargement of oral end of spermatophore, male paratype 5.7 mm ML, NSMT-Mo 85938. f gladius (part), unregistered specimen as in a. Scale bars: a = 20 μm; b, c, f = 1 mm; d, e = 0.1 mm. (Note: due to its delicate nature, the posterior end of the gladius beyond the rachis was damaged and lost during staining and mounting.)
Kodama jujutsu n. gen., n. sp. a–h, live animals photographed in the wild. i laboratory reared hatchling, dorsal view. j ventral view same specimen. The large white testis toward the posterior end of the mantle is clearly visible in images c, e and h. Prominent skin tags posterior to the eyes can be seen in c, e, g and h, and the nipple-like posterior tip of the mantle apparent in some postures is shown in a, c, and h. Curling and recurving the arms appears to be a common posture. Photos: a, c, d–h,
Common name: Hannan’s Pygmy Squid; Japanese name Tsuno-himeika.
Material examined
Type material
Holotype: Japan, Okinawa I, Hamamoto: 1♂, 6.0 mm ML, 26° 40′ 17.90′′ N, 127° 53′ 17.05′′ E, 24 Feb 2019, coll. C. Sugimoto and J. Jolly (NSMT-Mo 85932).
Paratypes: Japan: Okinawa I.: Miyagi I., 1♀, 6.8 mm ML, 26° 22′ N, 127° 59′ E, 23 Jan 2008, coll. N. Sato (NSMT-Mo 85935). Maeda Pt, 1♂, 3.5 mm ML, 26° 26′ 43.54′′ N, 127° 46′ 20.01′′ E, 26 Jun 2018, coll. J. Jolly and K. Asada (NSMT-Mo 85934). Onna Pt, 1♂, 5.3 mm ML, 26° 40′ 17.90.4′′ N, 127° 50′ 28.78′′ E, 24 Feb 2019, coll. K. Asada and C. Derup (NSMT-Mo 85933). Sunabe Sea Wall, 26° 19′ 41.09′′ N, 127° 44′ 35.98′′ E: 1♀, 8.7 mm ML (NSMT-Mo 85936); 1♀, 12.9 mm ML (NSMT-Mo 85937); 1♂, 5.7 mm ML, (NSMT-Mo 85938); 1♂, 6.4 mm ML (NSMT-Mo 85939); ♂, 6.0 mm ML (NSMT-Mo 85940), 24 Feb 2019, coll. B. R. Hannan.
Other material examined
Sunabe: 2 juv., 26° 19′ 41.09′′ N, 127° 44′ 35.98′′ E, 29 Mar 2019, coll. B. R. Hannan (NSMT-Mo 85941).
Diagnosis
As for genus.
Description
Counts and indices for individual specimens are given in Tables 6 (males) and 7 (females). Only mature specimens (five males and two females) were measured.
Males smaller than females: ML males 5.3–5.9–6.4 mm (SD 0.4), females 8.7–10.8–12.9 mm (SD 3.0). Mantle, short, rounded, blunt posteriorly (Fig. 7a, b) may be narrowed, nipple-like in live animals (Fig. 11a, c–e, h); MWI males 64.2–66.2–71.9 mm (SD 3.2), females 69.8–71.1–72.4 mm (SD 1.9). Ventral mantle margin straight to slightly concave (Fig. 7b). Fins rounded, maximum length approximately half mantle length, FLI males 29.7–38.7–43.9 mm (SD 5.4), females 48.1–49.9–51.7 mm (SD 2.6); positioned dorso-laterally on posterior third of mantle, FIIa males 62.3–67.7–78.1 mm (SD 6.1), females 54.3–59.3–64.4 mm (SD 7.1); fin width ~ 30% ML, FWI males 25.0–29.3–33.3 mm (SD 3.3), females 27.6–32.0–36.4 mm (SD 6.3); posterior margins curved; anterior margins with well-developed lobes, lateral lobes crescentic.
Funnel conical, base broad, tapered anteriorly (Fig. 7c); FuLI males 29.7–38.7–43.9 mm (SD 5.4), females 48.1–49.9–51.7 mm (SD 2.6); free for about 1/5 of its length, FFuI males 13.2–17.3–21.1 mm (SD 2.9), females 17.8–19.3–20.7 mm (SD 2.0). Funnel-locking cartilage (Fig. 7d), strait. Mantle-locking cartilage (Fig. 7e) compliments funnel member. Funnel valve small, flaplike, rounded anteriorly. Funnel organ, dorsal element broad, inverted V-shape with pointed anterior tip; ventral elements ovoid (Fig. 7f). Nuchal locking-cartilage (Fig. 7g) pronounced, deep, cylindrical, with raised margin of uniform width and median longitudinal furrow corresponding to rachis of gladius.
Head 40–50% ML, HLI males 46.7–50.7–53.1 mm (SD 2.6), females 37.2–38.7–40.2 mm (SD 2.1); slightly broader than ML in both sexes, HWI males 58.3.0–59.8–62.5 mm (SD 1.7), females 48.1–51.0–54.0 mm (SD 4.2). Eyes large, EDI males 11.3–13.9–15.8 mm (SD 1.7), females 12.4–13.7–14.9 mm (SD 1.8); ventral eyelids free. Eye covered by corneal membrane. Distinct, large olfactory pit on latero-posterior surface of head, posterior and ventral to eyes, close to mantle opening. Posterior-ventral to each eye is a prominent skin tag positioned ventrolaterally on the head. Lobes particularly prominent in live animals (Fig. 11b, c, e, g). Slightly anterior to eyes on ventral side of head are a pair of more prominent, small, rounded whitish projections.
Arms, broad basally, tapered distally, all similar length (particularly in females); arm formula usually 2.3.1.4 or 3.1.2.4 in males (Table 5), arm formula variable in females (Table 7). Arm length index of longest arm in males (ALI2) 26.7–37.9–47.2 mm (SD 7.6), females (ALI2) 25.2–32.7–40.2 mm (SD 10.6). All arms similar in shape, U-shaped in section. Sucker pedicels narrow. Chitinous inner ring of arm suckers without teeth, smooth or slightly crenulated on inner margin (Fig. 8a, b). Infundibulum with 4–5 rows of polygonal processes, innermost row of pegs elongate, cylindrical, with pegs decreasing in size toward outer margin of sucker, more elongate on one side of the sucker than the other (Fig. 8b); processes expanded, shallow dish-like distally contain tufts of low lobe-like papillae (Fig. 8c), outer-most sucker rim processes rectangular, tile-like, radially arranged, smooth, without papillae (Fig. 8b). Male and female arm suckers similar in size (Table 5). Sucker counts range from 18–25 on male normal arms, 15–24 in females. All arms connected by relatively shallow webs, protective membranes absent.
Male left ventral arm hectocotylised. Hectocotylus with 8.0–8.6–10.0 (SD 0.9) suckers proximally, remainder of arm without suckers; distal end of arm with large tongue-like flap attached dorso-laterally to arm a short distance proximal to distal arm tip (Fig. 9). Right ventral arm unmodified, with 8.0–8.6–10.0 (SD 0.9) suckers proximally (generally a greater number of suckers on left than on right ventral arm) remainder of arm without suckers. Left and right ventral arms similar in length (Table 5).
Tentacles slender, stalks naked, semicircular in section; oral surface convex. Club arm-like in form, just slightly narrower, cylindrical, tapers to blunt end distally (Fig. 8d); ClLI males 35.2–61.0–83.3 mm (SD 21.4) females 33.3–37.4–41.4 mm (SD 5.7). Sucker-bearing face of club only slightly convex. Suckers ~ 0.1–0.3 mm diameter in centre of club; arranged in two oblique transverse rows in both sexes. Total number of club suckers in males 24.0–25.6–28.0; females 34.0–34.5–35.0. Club sucker dentition (Fig. 3e): inner ring without teeth; infundibulum with 3–4 rows of polygonal processes; pegs narrow, elongate shallow, cup-like distally bearing papillae in depression. At periphery, pegs narrower and more elongate, with fewer papillae (Fig. 8f, g). Some inner pegs longer on inner side and recurved to cover depression. Outer-most sucker rim processes flattened, rectangular (Fig. 3f).
Gills with 15.0–15.4–16.0 (SD 0.5) lamellae per demibranch in males; 16.0–17.0–18.0 (SD 1.4) lamellae per demibranch in females.
Buccal membrane with six lappets and fringed inner margin; suckers absent. Radula with seven transverse rows of teeth (Fig. 10a). Rachidian teeth, broad rectangular basally, do not vary in shape along length of radula ribbon; teeth homodont, without cusps. First lateral teeth much smaller than rest, triangular, pointed, displaced toward second laterals; second laterals broad-based, much larger in size than first laterals and displaced toward midline. Marginal teeth narrow basally, scythe-like.
Upper beak (Fig. 10b) with short, triangular rostrum, hood curved; lateral margins with row of low teeth. Lower beak (Fig. 10c) with concave, finely toothed rostrum, flanked laterally by larger conical teeth. Distinct dark pigmentation restricted to rostrum and hood of upper and lower beaks.
Male reproductive tract (not illustrated) similar in structure to that of congeners. Spermatophores (Fig. 10d) approximately 1/5 mantle length; SpLI 18.3–20.3–22.6 mm (SD 2.2). Sperm reservoir simple, without coiled sperm cord. Cement body bipartite; aboral end elongate, cylindrical, narrows at oral end, connects to sperm reservoir via a narrow duct, connects via a narrow neck to long, narrower cylindrical portion leading to ejaculatory apparatus (Fig. 10e). Oral end of ejaculatory apparatus with 2–3 simple coils (Fig. 10d).
Female reproductive tract: Ovary large, occupies approximately half of mantle. Eggs of various sizes suggesting protracted multiple spawning. Ovary opens via single thick-walled oviduct at anterior end on left side of animal. Nidamental glands paired, broad, leaf-shaped located ventral to ovary toward, and overlying anterior half. Accessory nidamental glands absent. Eggs ovoid, 0.6–1.5 mm diameter; EgDI 6.9–9.3–11.6 mm (SD 3.3).
Gladius a thin, elongate, chitinous structure embedded in ventral side of dorsal mantle below adhesive pad; extending full length of mantle. Sclerotised rachis present (Fig. 10f).
Preserved animals cream with sparse dark purple chromatophores peppered evenly dorsally and ventrally on mantle, aboral side of arms and tentacles, and on ventral side of free portion of funnel. A row of dark chromatophores surrounds distal tip of funnel dorsally and ventrally. Single dark chromatophore on base of funnel on each side, anterior to tip of funnel-locking cartilage. Fins with evenly scattered chromatophores dorsally and ventrally, closest to junction with mantle. Outer rim of fins devoid of chromatophores. Chromatophores in a band dorsal to anus and in tissue overlaying viscera internally and in a patch on each side of anus. Between dark chromatophores are evenly scattered orange chromatophores.
Live animals with overall orange to yellow ‘base’ colouration. In some body patterns, chromatophores contract (appear large) at the same time on the head, in a transverse band toward the anterior end of the mantle and around the posterior end of the mantle (Fig. 10a, d).
Type locality
Japan, Okinawa I, Hamamoto: 1♂, 6.0 mm ML, 26° 40′ 17.90′′ N, 127° 53′ 17.05′′ E.
Distribution
Japan, Okinawa Island from Miyagi I., 1♀, 6.8 mm ML, 26° 22′ N, 127° 59′ E to Sunabe 26° 19′ 41.09′′ N, 127° 44′ 35.98′′ E. Visual observations made by Brandon Hannan (personal communication) extend the northernmost extent of the range to 26° 30′ 43.02′′ N, 127° 52′ 07.92′′ E. Depth range 1–20 m.
Habitat and biology
Kodama jujutsu is found around coral reefs and has been seen hunting and foraging after sunset, often in open water near reef cuts. Underwater naturalist and photographer, Shawn Miller, has captured a typical, possibly defence posture (Fig. 10a, c–h) in which the animal spreads the arms and sometimes the tentacles out widely in a circle surrounding the mouth, with the distal tips of these appendages curved inwards. Sometimes the arms are extended dorsally above the head. This can be accompanied by the contraction of the mantle posterior to the fins forming a rounded nipple-like tip. They readily follow shrimp attracted to dive camera lights or torches at night. The species has also been found in shallow seagrass beds.
It first attracted Brandon Ryan Hannan’s attention in 2019 when he noticed the unusual protrusions below the eyes and observed the species attaching itself to the underside of coral or any underwater substrate. At the popular Sunabe Seawall dive site in Okinawa he observed K. jujutsu attached to the undersides of various hydroids to which the hydroid nematocysts (stinging cells) do not deter K. jujutsu. A further interesting observation was made in 2019 of the pygmy squid attached to a hydroid that the swimming nudibranch, Bornella anguilla S. Johnson, 1984 was eating (Fig. 12a). When the nudibranch reached the end of the hydroid it did not touch the squid and the squid didn’t move. Clearly the squid was not concerned about the nudibranch—testament to the fact that nudibranchs are generally highly specialised feeders. In this case, the hydroid, and not the pygmy squid is the target for B. anguilla. (The species is known to extend its buccal bulb and hydroid branches are drawn into the mouth to be stripped of its polyps.)
The species has been observed numerous times during diving around Okinawa at night and sometimes at dusk in water with temperatures ranging between 20 and 27 °C. When observed eating, the prey was always small shrimp with bodies smaller or similar in size to themselves (B. Hannan, personal observations, Fig. 12b, c).
Etymology
The specific name jujutsu is derived from the Japanese word jūjutsu that is a martial art of the same name, translating to ‘gentle art’. The goal of the sport is to control your opponents by grappling them. This pygmy squid has been seeing grappling shrimp in a similar fashion. The name is used as a noun in apposition.
Remarks
Traits that separate K. jujutsu from X. notoides and Idiosepius are described in the generic diagnosis above. The character combination that separates Kodama jujutsu from individual Idiosepius spp. are tabulated in Table 8.
Some females have spermatophores embedded at the base of the ventral arm pair. The 3.5 mm ML male specimen from Maeda Point has well-developed spermatophores indicating this species matures at a very small size.
Behaviour in captivity
Idiosepius kijimuna and K. jujutsu habitually attach to substrate including aquarium plants and tank walls where they remain unless swimming to change positions, hunt, or mate. Both species were observed to attack the dorsal side of prey to immobilise it within seconds and both swam or attached to substrate while consuming prey (Online Resource 1, MOESM1; Fig. 5b). Adults of both species swam by pumping water through the funnel and moving the fins in a high frequency figure-eight motion. Idiosepius kijimuna swam in sudden and quick movements, sometimes swinging the mantle in a bobbing motion with the arms recurved or positioned above the head. The mantle is often inflated, and rounded and the posterior end is constricted, making a nipple-like projection (Online Resource 2, MOESM2 and Fig. 11). The arms of Kodama jujutsu were usually splayed outwards and above the head and the tip of the ventral mantle was produced into a nipple. Sometimes while swimming, the head and arms appeared fixed as the body moved in a vertical bobbing motion. In this way the outline of the animal is disrupted perhaps making it look more like a bit of floating debris. Idiosepius kijimuna exhibits a more streamlined and controlled swimming motion (Online Resources 3 MOESM3, 4 MOESM4; Fig. 5a, c). Prior to mating, Idiosepius kijimuna males approached females head on and mating occurred in the head-to-head position (Online Resource 5, MOESM5). The males appear to deposit the spermatophores on the oral side of the arm crown, perhaps in the buccal region. In contrast, Kodama jujutsu males approached the female from below (i.e., both facing the same direction) and extended the hectocotylus toward the female, appearing to deposit spermatophores on the ventral side of the head (Online Resource 6, MOESM6). Both sexes exhibited a row of two white papillae posterior-laterally on either side of the mantle and on the ventral side of the head when mating and approaching to mate. (While assuming the hectocotylus in both species was used to deposit spermatophores, it is difficult to determine from the video footage whether the hectocotylus or the tentacles are being used to place them.) One specimen captured in Okinawa at Mizugama (north wall) laid fertile eggs that successfully hatched one week after capture. As there were no males present, clearly the sperm had been stored by the female for at least one week.
Discussion
Kodama jujutsu is sister to a clade containing X. notoides and all Idiosepiidae. It shares a number of morphological traits with the southern Australian endemic species, Xipholeptos notoides. Most significantly, both species have a straight, narrow funnel-mantle locking cartilage and the gladius extends along the full length of the mantle and has a distinct medial rachis. The presence of a fully developed gladius with sclerotised rachis seems to be a primitive trait in this family that is lost in the genus Idiosepius in which the gladius is reduced to a chitinous shield. Kodama jujutsu and X. notoides clearly differ, however, in some other morphological traits, and are clearly separated on molecular grounds.
The spermatophore transfer method shown by I. kijimuna is similar to that of I. thailandicus (Nabhitabhata and Suwanamala 2008) and differs from I. paradoxus (Ortmann, 1888), where males transfer spermatophores while grasping females (Sato et al. 2013). The mating behaviour of X. notoides has not yet been described. Given that K. jujutsu and X. notoides do not have dimorphic hectocotyli, it may be that they exhibit similar mating behaviour that differs from that of Idiosepius spp., with males approaching the females from below. Observation of the deposition of spermatophores in all idiosepiids using high speed photography as used in Sato et al. (2013) would be valuable to compare strategies. In addition, it would be useful to re-confirm whether I. thailandicus Chotiyaputta et al. (1991) uses the tentacles for spermatophore transfer as reported by Nabhitabhata and Suwanamala (2008).
Very apparent from this study is evidence of the importance of live animal observations in defining taxa within this family. As these tiny cephalopods are easy to culture in captivity, and are found at depths readily accessible to divers, future behavioural studies and observations will undoubtedly yield many useful insights to aid our understanding of this particularly enigmatic group of tiny cephalopods.
Data/code availability
Genetic sequence data are available from GenBank, https://www.ncbi.nim.nih.gov/genbank/.
References
Abbo LA, Himebaugh NE, DeMelo LM, Hanlon RT, Crook RJ (2021) Anaesthetic efficacy of magnesium chloride and ethyl alcohol in temperate octopus and cuttlefish species. J Am Assoc Lab Anim Sci 60:556–567. https://doi.org/10.30802/AALAS-JAALAS-20-000076
Allcock AL, Strugnell JM, Johnson MP (2008) How useful are the recommended counts and indices in the systematics of the Octopodidae (Mollusca: Cephalopoda). Biol J Linn Soc 95:205–218. https://doi.org/10.1111/j.1095-8312.2008.01031.x
Fiorito G, Affuso A, Basil J, Cole A, de Girolamo P, D’Angelo L et al (2015) Guidelines for the care and welfare of cephalopods in research -a consensus based on an initiative by CephRes, FELASA and the Boyd group. Lab Anim 49:1–90. https://doi.org/10.1177/0023677215580006
Guindon S, Gascuel O (2003) A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. https://doi.org/10.1080/10635150390235520
Hall NE, Hanzak J, Allcock AL, Cooke IR, Ogura A, Strugnell JM (2014) The complete mitochondrial genome of the pygmy squid Idiosepius (Cephalopods: Decapodiformes), the first representative from the family Idiosepiidae. Mitochondrial DNA Part A DNA Mapp Seq Anal Early Online 27:5–6. https://doi.org/10.3109/19401736.2013.865180
Hasegawa M, Kishino H, Yano T (1985) Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 22:160–174. https://doi.org/10.1007/BF02101694
Jolly J, Hasegawa Y, Sugimoto C, Zhang L, Kawaura R, Sanchez G, Gavriouchkina D, Marle´taz F, Rokhsar D (2022) Lifecycle, culture, and maintenance of the emerging cephalopod models Euprymna berryi and Euprymna morsei. Front Mar Sci 9:1039775. https://doi.org/10.3389/fmars.2022.1039775
Katoh M, Kuma M (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066. https://doi.org/10.1093/nar/gkf436
Lanfear R, Calcott B, Ho SYW, Guindon S (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol 29(6):1695–1701. https://doi.org/10.1093/molbev/mss020
Nabhitabhata J, Suwanamala J (2008) Reproductive behaviour and cross-mating of two closely related pygmy squids Idiosepius biserialis and Idiosepius thailandicus (Cephalopoda: Idiosepiidae). J Mar Biol Ass UK 88:987–993. https://doi.org/10.1017/S0025315408001616
Nguyen L-T, Schmidt HA, von Haeseler A, Quang Minh B (2015) IQ-Tree: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. https://doi.org/10.1093/molbev/msu300
Ogden BE, Pang W, Agui T, Lee BH (2016) Laboratory animal laws, regulations, guidelines and standards in China mainland, Japan, and Korea. ILAR J 57:301–311. https://doi.org/10.1093/ilar/ilw018
Reid A, Strugnell J (2018) A new pygmy squid, Idiosepius hallami n. sp. (Cephalopoda: Idiosepiidae) from eastern Australia and elevation of the southern endemic ‘notoides’ clade to a new genus, Xipholeptos n. gen. Zootaxa 4369(4):451–486. https://doi.org/10.11646/zootaxa.4369.4.1
Roper CFE, Voss GL (1983) Guidelines for taxonomic descriptions of cephalopod species. Mem Natn Mus Vic 44:48–63. https://doi.org/10.24199/j.mmv.1983.44.03
Sato N, Yoshida M-A, Fujiwara E, Kasugai T (2013) High-speed camera observations of copulatory behaviour in Idiosepius paradoxus: function of the dimorphic hectocotyli. J Moll Stud 79(2):183–186. https://doi.org/10.1093/mollus/eyt005
Stamatakis A (2014) RAxML Version 8: a tool or phylogenetic analysis and post-analysis of large phylogenies. Bioinform 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web-servers. Syst Biol 75(5):758–771. https://doi.org/10.1080/10635150802429642
Strugnell JM, Hall NE, Vecchione M, Fuchs D, Allcock AL (2017) Whole mitochondrial genome of the Rams’ Horn squid shines light on the phylogenetic position of the monotypic order Spirulida (Haeckel, 1896). Mol Phyl Evol 109:296–301. https://doi.org/10.1016/j.ympev.2017.01.011
Trifinopoulos J, Nguyen L-T, von Haeseler A, Quang Minh B (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232-235. https://doi.org/10.1093/nar/gkw256
von Byern J, Klepal W (2010) Re-evaluation of taxonomic characters of Idiosepius (Cephalopoda, Mollusca). Malacologia 52(1):43–65. https://doi.org/10.4002/040.052.0104
von Byern J, Söller R, Steiner G (2012) Phylogenetic characterisation of the genus Idiosepius (Cephalopoda; Idiosepiidae). Aquat Biol 17:19–27. https://doi.org/10.3354/ab00445
Acknowledgements
Many thanks to Hiroaki Fukumori who sent AR specimens of I. kijimuna that were first sequenced and recognised as an undescribed idiosepiid in Reid and Strugnell (2018). We thank Keishu Asada for noticing K. jujutsu was likely an undescribed species and for his support early in the project via sampling and photography. Thanks to Brandon Ryan Hannan, Rob Kidston, Matt Rudolph, Christian Drerup, and Shawn Miller for their tremendous contributions photographing and collecting specimens of K. jujutsu. Brandon Hannan also generously shared some fascinating behavioural observations that we have included. Thanks to Masahiro Hirano, Zdenik Lajbner, Ryuta Nakajima, Kaname Sasaki, Chikatoshi Sugmimoto and Ryoko Yanagisawa for collecting specimens of I. kijimuna. Thanks to the molecular genetics unit at OIST for supporting this project, especially Professor Daniel Rokhsar for his overall support of the unit, Lin Zhang for her support in culturing these animals, and Chikatoshi Sugimoto for his assistance with sampling and knowledge of Okinawan cephalopods. We thank the Marine Climate Change Unit, particularly Professor Timothy Ravasi for supporting the project and Yoko Shintani for her logistical support. We are grateful to Sue Lindsay at Macquarie University, Sydney for assistance with Scanning Electron Microscopy and to Melissa Joyce for her assistance in preparing Figure 1. Finally, many thanks to the reviewers for their thorough appraisal of this manuscript and very helpful comments that resulted in its improvement.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funding for this project was provided by the Okinawa Institute of Science and Technology (OIST) to the Molecular Genetics and the Marine Climate Change Units.
Author information
Authors and Affiliations
Contributions
NS and JJ: led the sample collection, fixed animals, extracted DNA and sequenced specimens. JS: analysed the molecular data and generated the phylogenetic tree. JJ: managed the culture of live animals. AR: wrote the species descriptions and prepared Figs. 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12. All authors contributed to the writing and the final editing of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical approval
Cephalopods are not covered under the Japanese legislation ‘Act on Humane Treatment and Management of Animals’ (Ogden et al. 2016). Procedures and rearing protocols followed the guidelines set by Directive 2010/63/EU for cephalopods (Fiorito et al. 2015) and animal welfare guidelines set by OIST Animal Care and Use Committee. The highest quality of care was taken to reduce the suffering of animals.
Additional information
Responsible Editor: R. Rosa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
[Version of record, published online 21 October 2023; http://zoobank.org/urn:lsid:zoobank.org:pub:EAAF7947-9DE4-4958-8FAC-44A0E431FB8C].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 Kodama jujutsu eating a shrimp while attached to a solid substrate © J. Jolly. (MOV 123840 KB)
Supplementary file2 Kodama jujutsu swimming. © J. Jolly. (MOV 66879 KB)
Supplementary file3 Idiosepius kijimuna swimming. © J. Jolly. (MOV 158591 KB)
Supplementary file4 Idiosepius kijimuna female (foreground) and male (background) swimming. © J. Jolly. (MOV 48412 KB)
Supplementary file5 Idiosepius kijimuna mating. The male is on the left and the larger female on the right. © J. Jolly. (MOV 69527 KB)
Supplementary file6 Kodama jujutsu mating (male on the left, below the female). © J. Jolly. (MOV 70948 KB)
Appendix
Appendix
See Table 9.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reid, A., Sato, N., Jolly, J. et al. Two new pygmy squids, Idiosepius kijimuna n. sp. and Kodama jujutsu n. gen., n. sp. (Cephalopoda: Idiosepiidae) from the Ryukyu Islands, Japan. Mar Biol 170, 167 (2023). https://doi.org/10.1007/s00227-023-04305-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04305-1