Abstract
Dive studies across mammals, birds, reptiles and fish often focus on deep dives, and shallow water diving has tended to be overlooked. For air-breathers, foraging in shallow water poses challenges since the lungs generate buoyancy, and shallow divers must trade off the extent of inhalation against the negative buoyancy needed to avoid floating to the surface. Using high-resolution depth loggers, we addressed this knowledge gap around the ecology of shallow water diving at a foraging site for hawksbill turtles (Eretmochelys imbricata) where depth was typically < 3 m. Contrary to predictions, dive durations were long, particularly at night (mean dive duration per turtle: 17–61 min, n = 12 turtles, n = 2576 nocturnal dives), despite warm water temperatures (24–37 °C). Dive efficiency (% time submerged) for hawksbills was 98%, the highest recorded for any air-breathing marine vertebrate including penguins (60–78%), seals (51–91%), cetaceans (68–87%), and other sea turtle species (68–95%). Hawksbills usually dive for much longer (42–286% increase) than green and loggerhead turtles when depth and temperature are accounted for. Hawksbill turtles likely forage in very shallow water to reduce predation risk from sharks: of 423 hawksbills captured by hand, none had any evidence of shark attack, although large sharks were present in nearby deeper water. Our results challenge the prediction that shallow water dives by air-breathers will usually be short and open the way for comparative studies of the ecology of shallow water diving in a range of other taxa. Our work emphasises the likely importance of predation risk in shaping patterns of habitat utilisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For marine species, patterns of depth utilisation play a central role in their ecology and influence foraging success, risk of predation and energy balance (e.g. Sims et al. 2005; Heithaus et al. 2007; Teo et al. 2007). For air-breathing animals that forage underwater, maximising the time spent at foraging depth maximises the energetic return per unit foraging effort (Weise et al. 2010). Minimising time spent at the surface additionally reduces the risk of being predated upon for many species (Heithaus and Frid 2003). While there is a huge amount of work on patterns of depth use across a broad range of taxa including fish, seabirds (e.g. penguins), marine mammals (e.g. seals and whales) and reptiles (e.g. turtles and sea snakes), these studies tend to focus on patterns of deeper diving to 10s or 100s of metres (e.g. Williams and Ponganis 2021), and the ecology of shallow water diving has tended to be neglected (but see Sims et al. 2005). For the deepest divers, such as sperm whales (Physeter macrocephalus) and elephant seals (Mirounga spp), adaptations to deep diving (100s of metres) include the primary oxygen stores being in the blood (haemoglobin) and muscle (myoglobin), with animals exhaling prior to submergence (Fahlman et al. 2017). Conversely, for shallower divers routinely descending to only a few 10s of metres, such as sea otters (Enhydra lutris), dolphins and cheloniid (hard-shelled) turtles, the lungs may be an important oxygen store for dives (Lutcavage and Lutz 1997).
Many breath-hold divers have been shown to adjust inspired air volume according to the depth of the coming dive in order to regulate the buoyancy created by air in the lungs (penguins: Sato et al. 2002; sea turtles: Hays et al. 2004; freshwater turtles: Peterson and Gomez 2008; sea lions: McDonald and Ponganis 2012). As gas compresses with depth due to increasing pressure, such divers are able to dive with a greater inspired lung volume when travelling to greater depths while still achieving neutral or negative buoyancy at the target depth. As a result, both the oxygen store and the duration of dives are expected to increase with depth for those divers using the lungs as an important portion of their oxygen store. In line with predictions, dive duration has been shown to increase with depth in multiple taxa (e.g. sea turtles: Hochscheid et al. 1999; dugongs Dugong dugong: Churchward 2001; penguins: Wilson 2003), with the strongest effect seen in sea turtles (Hays et al. 2004) which are the most lung-dependent of these divers (lung oxygen store as a percentage of total bodily oxygen stores—penguins: 19–53%, Ponganis and Kooyman 2000; sirenia: 33%, Ponganis 2011; cheloniids: 72%, Lutz and Bentley 1985).
The dual functionality of the lungs as both oxygen store and buoyancy regulator (Hochscheid et al. 2003) therefore presents a challenge for divers inhabiting very shallow environments. Shallow diving should be relatively inefficient, with individuals having to resurface after short intervals due to the limited lung oxygen store needed to remain passively submerged in shallow water (Matley et al. 2020). To date there has been relatively little consideration of dive duration during very shallow water diving by air-breathing species, although the available data largely support the prediction of short dives. Short dives were recorded in foraging sea turtles and dugongs inhabiting shallow lagoons in Greece and Australia, with mean dive durations of 2–4 min at 2 m depth in adult green (Chelonia mydas) and loggerhead turtles (Caretta caretta; Houghton et al. 2000; Thomson et al. 2012) and a mean duration of 3 min at 5 m mean depth in dugongs (Chilvers et al. 2004). Short dives were also observed in internesting green turtles in a shallow estuarine habitat in French Guiana, with the majority of dives < 5 min and < 2 m (Chambault et al. 2016). Elsewhere in Australia, however, green turtles have been recorded diving for over 20 min in shallow water (2–6 m depth, 26–29 °C: Hazel et al. 2009). Here, we use high-resolution, fast sampling depth loggers to assess the depth utilisation behaviour of a foraging population of hawksbill turtles within a shallow lagoonal habitat in the Chagos Archipelago, Indian Ocean. We calculate standard metrics of dive efficiency (Watwood et al. 2006; Schagatay et al. 2011), (i) the % of time spent submerged and (ii) the % of the dive cycle spent in the bottom phase of dives, that we then compare against other sea turtle species as well as diving mammals, birds and reptiles often diving to 10s or 100s of metres. In this way, we assess the relative performance of shallow diving hawksbill turtles.
Materials and methods
Recording dive behaviour
High-resolution time-depth recorders (TDRs) were deployed on hawksbill turtles foraging in Turtle Cove at the southern tip of the horseshoe-shaped lagoon of Diego Garcia atoll in the Chagos Archipelago, Indian Ocean (7.42 °S, 72.46 °E), during October 2012 (n = 5) and February to March 2021 (n = 7). Turtles were captured at low tide in shallow water (< 1 m) by approaching slowly and carefully from behind before seizing the carapace, whereupon turtles were carried ashore. The curved carapace length (CCL) and curved carapace width (CCW) were measured using a flexible tape, and mass recorded with a suspended scale (Pesola Macro line 50 kg scale, Pesola AG, Switzerland). Maximum precision TDRs (G5 data loggers, 10 bar sensors with 0.03 m resolution, Cefas Technology Limited, Lowestoft, UK) were attached proximally to the trailing edge of flippers, using wire ties to fix them onto flipper tags placed at the thin junction between adjacent thick flipper scales (Supplementary Information Fig. S1). The TDR data sampling rate was every 1–5 s for pressure/depth and every 30–60 s for temperature. TDRs were recovered after various intervals when the turtles were next encountered in the cove (SI Table S1).
Analysis of dive data
The first four hours of each deployment were excluded in case of altered behaviour following capture and attachment (Thomson et al. 2012). Overall data analysis and visualisation were performed in R (R Core Team 2021). Zero-offset correction was scripted directly in base R. Individual dive characteristics and post-dive surface intervals (PDSI) were measured using MultiTrace Dive (Jensen Software Systems, Hamburg, Germany) with a minimum duration of 5 min and a threshold depth of 0.2 m, as has been described for shallow divers by Hays et al. (2007).
Welch’s t test was used to test for differences in diurnal vs. nocturnal dive depth and dive duration for individual turtles, and repeated measures ANOVA was used to test for a diel difference in dive duration at the group level. Linear modelling was used to explore relationships between dive depth and dive duration, and temperature and dive duration, and was performed in R using the package ‘lm’, as well as the ‘MASS’ package for Boxcox transformation. Inverse transformation (1/duration) was used to rectify skew in the residuals for two models (those for diurnal dive duration). Results for subsetted datasets are reported to minimise co-variant effects: mid-temperature dive datasets exclude dives < 28 °C and ≥ 30 °C, and dives by immature turtles exclude data from the single subadult turtle.
Sequential dives were considered to be independent as each dive is separated by a surface interval. However, all statistical tests and linear models were repeated with reduced datasets consisting of every other dive per individual.
Evidence of shark attacks
Between 2012 and 2021, hawksbill turtles were captured in the shallows of the lagoon (n = 423) and examined for any evidence of recent shark attack such as scars on the carapace or skin, or missing parts of flippers. Recent injuries were noted when they were a different colour from surrounding flesh.
Comparisons across taxa: sea turtles
Sea turtle dive profiles are often categorised by shape, so comparisons were made specifically for U-shaped dives in order to minimise variability in activity levels across dives. U-shaped dives or U-dives have a steep descent followed by a flat bottom phase and a steep ascent, and are associated with bottom feeding and resting (Hochscheid 2014). Comparative data points were collected from the literature where individual or group mean depth and duration were provided for U-shaped dives in non-migratory cheloniid sea turtles in warm waters. Where mean data included a variety of dive types, depth and duration data were instead taken for flat-bottomed U-shaped dives shown in example dive profile figures, to allow inclusion of studies not providing mean data specific to U-dives. Where dives were classified into active/foraging and resting U-dives (e.g. Ballorain et al. 2013), or Type 1a (flat-bottomed U-dives) and Type 1b dives (U-dives with variability in bottom phase depth; Houghton et al. 2002), data were included for resting U-dives/Type 1a only. Where temperature categories were given, only the warmest category was included to enable comparison to conditions in Diego Garcia. See SI Section S1. Linear-plateau regression models were fitted using the nls package in R (R Core Team 2021) with a custom ‘if-else’ function describing the linear-plateau model (Gradcylinder.org 2022). Where nls failed to choose a plateau value (hawksbill turtles only), the most conservative model with the lowest R2 value was chosen. This value also matched the quadratic-plateau model that successfully fitted using nls; however, the quadratic term did not significantly enhance the model and so was not included.
Comparisons across taxa: diving vertebrates
Diving efficiency is discussed in the literature in relation to either the proportion of time spent in the bottom phase of dives (e.g. Watwood et al. 2006), or the percentage of time spent submerged versus at the surface (e.g. Schagatay et al. 2011). Here, we present data for both measures of dive efficiency in foraging hawksbill turtles and compare our findings with data sourced from published studies of other air-breathing marine vertebrates. Dive efficiency in terms of the proportion of time spent in the bottom phase of dives varies with dive type and the depth an animal must travel to in order to reach the bottom phase of dives. We therefore compare dive efficiency across taxa using percent time spent submerged, to broaden comparability and to remove depth-bias.
Results
Diving performance of hawksbill turtles
Dive data were recorded for twelve hawksbill turtles (CCL: 36.2–70 cm, mass: 3.7–29.5 kg) for a total of 124 days (median: 5.0 d per individual, range: 0.6–51 d). The majority of dives were U-shaped dives, with occasional V- or W-shaped dives (see Fig. 1a, b for example depth profiles). A total of 7458 U-shaped dives of over five-minute duration were extracted from the dive records (median: 285 dives per individual, range: 23–3516; Fig. 1, SI Table S1; Stokes et al. 2023). Often the depth of consecutive dives paralleled the change in tidal height, i.e. dives became deeper as the tide came in and vice versa (Fig. 1a, b). Even though the threshold depth for recording a dive was very shallow (0.2 m), due to the high sensor resolution even these shallow dives were clearly evident in the dive records and were often many minutes long (Fig. 1b).
a An example 24 h depth profile for a hawksbill turtle. Blue dot-dash shows tide heights at the lagoon mouth as calculated using the rule of twelfths from high and low tide times (shown in red). b An example 4 h depth profile showing a bout of very shallow diving (0.25–0.5 m), with U-shaped dives up to 22 min clearly delineated (depth changes with tidal height). Distributions of c mean bottom depth of dive d dive duration e mean ambient temperature during dive and f post-dive surface intervals for n = 7458 U-shaped dives. Depth use had similar distribution across diurnal and nocturnal dives (panel c), while dive duration is markedly longer in nocturnal compared to diurnal dives (panel d). Blue bars: diurnal dives, red: nocturnal dives, purple: overlap between diurnal and nocturnal dives (c–e) or all dives (f)
Mean bottom depth of U-dives for each individual ranged from 0.55–1.52 m for diurnal dives (mean ± SD: 0.86 ± 0.41 m) and 0.37–2.38 m for nocturnal dives (mean ± SD: 0.81 ± 0.47 m), i.e. turtles exhibited shallow dives both day and night (Fig. 1c; SI Table S1). Diurnal dives were significantly shorter (mean ± SD, range in means per individual: 9 ± 5 min, 7.3–21.8 min) than nocturnal dives (24 ± 11 min, 17.1–60.9 min; repeated measures ANOVA, F(1,11) = 49.72, p < 0.001; Fig. 1d; SI Table S1). This was also true for 11 out of 12 turtles when testing at the individual level (Welch’s t test, p < 0.001, S1 Table S1), the one non-significant result being due to the low number of dives recorded (n = 3 diurnal U-dives, n = 20 nocturnal U-dives). Post-dive surface intervals (PDSI) were consistently short (Fig. 1f), with mean PDSI per individual ranging between 0.1 and 0.5 min (SI Table S1). So the mean PDSI for each individual was much shorter than the mean dive duration, with this ratio ranging from 0.01 to 0.03 across the 12 individuals.
Dive duration increased with depth as expected, particularly at night (Fig. 2a, Table 1). Although turtles occupied a very narrow range of depths, they experienced a large variation in ambient temperature (Figs. 1c, e, and 2b). For dives to < 1 m the ambient temperature varied widely between 24 and 37 °C, and dive duration decreased markedly with increased temperature (Fig. 2c, Table 1). For example, diurnal and nocturnal dives at 25 °C averaged 9 and 32 min respectively, decreasing to 6 and 13 min at 32 °C (Fig. 2c).
Dive depth, dive duration and ambient temperature for hawksbill turtles foraging in Diego Garcia lagoon. a Mean bottom depth and duration of mid-temperature U-dives by immature turtles. b Mean ambient temperature during dive versus mean bottom depth for U-dives. In the shallowest water there was a much greater range of temperatures. c Dive duration during U-dives as a function of ambient temperature. Diurnal dives are shown in blue, nocturnal in black. Regression equations are given in Table 1
Diel differences in depth use were not consistent among turtles, i.e. some individuals performed deeper dives during the day (n = 6) and others during the night (n = 3; Welch’s t test, p < 0.05, SI Table S1). All statistical tests and linear models that were significant with all dives included remained significant when repeated with every second dive removed, other than differences in mean diel depth for two turtles (T6 and T10) that were significant to p < 0.05 with all dives included (those with a single asterisk for significance level in SI Table S1). Diel depths were significantly different for 7 out of 12 turtles when using the reduced dataset, compared to 9 out of 12 turtles when using the full dataset (SI Table S1).
Evidence of shark attacks
In an estimated 190 h of in-water captures of turtles in this shallow lagoon environment we frequently observed small piscivorous sharks, namely small black tip sharks (Carcharhinus limbatus) and sickle fin lemon sharks (Negaprion acutidens), which do not predate on turtles. No larger sharks or other species of shark were seen in this shallow area of the lagoon. For 423 hawksbill turtles captured and assessed, there was no evidence of any recent shark injuries.
Comparisons across taxa: sea turtles
When comparing U-dive depths and durations from the current study to those from other published sources, hawksbill dive durations were longer for any given depth under 25 m than loggerhead or green turtle dive durations, with a clear separation of dive data by species (Fig. 3, SI Table S2). Modelled dive duration was 42–286% higher for hawksbills when compared to green and loggerhead turtles (equations given in Table 2). For example, for dives to 1, 5, and 10 m the fitted models gave mean dive durations of around 20, 33 and 49 min for hawksbill turtles, 7, 15 and 25 min for greens and < 1, 15 and 32 min for loggerheads.
Depth and duration of cheloniid U-shaped dives. Eleven immature and one subadult hawksbill turtle in Turtle Cove, Diego Garcia (white squares), against other cheloniid populations sourced from published literature: mean depth and duration of U-shaped dives per individual or per study group. Red: hawksbill turtles (white distinguishes current study hawksbill turtles), green: green turtles, black: loggerhead turtles, yellow: flatback turtle; circles: adults, squares: juveniles, triangles: mixed adults and juveniles. Regression equations given in Table 2
Comparisons across taxa: diving vertebrates
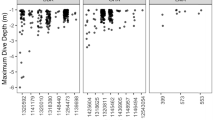
Dive efficiency was found to be consistently high across all individuals (percent time spent submerged: mean ± SD, range in means per individual: 98.1 ± 0.02, 96.6–99.6%; percent time in bottom phase of dives: 93.4 ± 0.04, 92.4–97.5%; SI Table S1). Dive efficiencies recorded here are high compared to previously published data for reptile, bird and mammal breath-hold divers (Fig. 4, SI Table S3). For example, among marine mammals including phocid and otariid seals, cetaceans, the walrus (Odobenus rosmarus) and humans, dive efficiency ranged from 50 to 91%, being highest in hooded seals (Cystophora cristata). Among seabirds, dive efficiency ranged from 43 to 78% and was highest in the gentoo penguin (Pygoscelis papua). Among marine reptiles, dive efficiency ranged from 68 to 98% and was highest in hawksbill turtles from the current study.
Discussion
There is a wide body of literature showing that many marine vertebrates, including air-breathers, often reside in shallow water for a multitude of reasons including foraging, resting, breeding and migration (e.g. Sims et al. 2005; Cerritelli et al. 2022). It might be expected that shallow dives in air-breathers come with a trade-off of short duration, due to the constraints on inhalation as part of lung-controlled buoyancy regulation (Hays et al. 2004). However, our results are noteworthy in showing that highly efficient diving is possible for a breath-hold diver on very shallow dives, and hence the habitat of shallow water seabeds can be occupied on long dives.
Most studies of diving in air-breathing marine vertebrates consider dives to 10s or 100s of metres and so generally the threshold depth for defining a dive is typically 2–5 m (e.g. Weise et al. 2010; Zimmer et al. 2010), i.e. much deeper than used here (0.2 m). This selection of a dive threshold works well for deeper diving animals, where often the pressure sensor used will have much greater measurement range, often capable of recording dives to 1000 m or more (e.g. Houghton et al. 2008). As a consequence, these sensors have lower precision for recording near-surface activity. In contrast, the depth loggers we used have very high resolution (3 cm) but have a limited measurement range, with a maximum of about 100 m in our case. These sorts of high-resolution depth loggers have been shown to reliably record shallow diving and have been used, for example, in studies with semi-aquatic divers such as mink swimming in rivers (Hays et al. 2007; Harrington et al. 2012), where dives were similarly defined as excursions to > 0.2 m.
Hawksbill turtles, like many fish (e.g. Sims et al. 2005), marine birds (Grémillet et al. 1998) and marine mammals, such as dugongs and manatees (Chilvers et al. 2004), likely occupy the seabed for foraging and/or resting. Certainly the U-dives we recorded are indicative of benthic diving, as has been noted in a number of other sea turtle studies (Seminoff et al. 2006; Thomson et al. 2011). This inference of benthic dives from the dive shape is further supported by the often close parallel between the depth of consecutive dives and the change in tidal height, i.e. the turtles essentially provided the same sort of data as a tide gauge. Frequent dives to the seabed are expected in hawksbill turtles, as well as other sea turtles, since they both rest and feed on the seabed (Houghton et al. 2003). For example, elsewhere hawksbill turtles have been documented feeding on sponges on coral reefs (van Dam and Diez 1997), while a complete lack of motion associated with benthic sleeping has been shown for hawksbill turtles in the Seychelles (Houghton et al. 2003). Consistent with other studies of cheloniid turtles (e.g. Makowski et al. 2006; Witt et al. 2010), we observed longer U-dives at night indicative of benthic resting, with the longer dives likely facilitated by the lower metabolic rate of quiescent turtles compared to those foraging during the day. In contrast with studies finding a consistent pattern of diel depth use across turtles (e.g. deeper nocturnal depth use: green turtles, Ascension Island, Hays et al. 2002a, b; green turtles, Florida, Makowski et al. 2006; deeper diurnal depth use: green turtles, Hawaii, Brill et al. 1995; hawksbill turtles, Cayman Islands, Blumenthal et al. 2009), turtles at this site selected nocturnal depths that were either deeper or shallower than those they occupied during the day, depending on the individual turtle.
As U-dives are generally to the seabed, they give a good idea of the bathymetry encountered during diving. Hence, we can confidently conclude that the equipped hawksbills occupied very shallow depths (typically shallower than 3 m). This is in line with GPS tracking from this lagoon that has shown long-term occupation of very shallow areas (Hays et al. 2021). Certainly the instrumented turtles had ready access to deeper water, but they chose not to occupy these areas. For example, within the Diego Garcia lagoon only a few km from where animals were equipped there is deeper water (max depth 31 m) with abundant sponge and coral cover (Hamylton and East 2012 Fig. 7), i.e. the types of preferred foraging habitat for this species. Hence, we conclude that occupation of shallow water by the turtles was through choice, rather than simply a consequence of limited available foraging habitat.
Depth selection in sea turtles and other marine vertebrates may often be linked to forage availability, maximising resting time, thermoregulation and avoiding predation (Hays et al. 2002a; Heithaus et al. 2007; Schofield et al. 2009). In our study one possibility is that the extremely shallow water leads to the exclusion of large sharks and so creates a low predation risk foraging environment for hawksbill turtles. Our evidence supports this possibility, with no large sharks seen in the shallow areas occupied by the equipped turtles, even though large sharks are found in deeper areas nearby (Winterbottom and Anderson 1997; Dunn et al. 2022). Furthermore, none of hundreds of captured hawksbill turtles showed any evidence of shark injuries, in contrast to other locations where sea turtles are frequently observed with missing rear flippers consistent with shark predation (Heithaus et al. 2002; Hill et al. 2017). So our observations are consistent with very shallow water offering hawksbill turtles a refuge from shark predation and, as such, our findings add to the growing body of evidence for the importance of predators in shaping habitat selection (Heithaus et al. 2008; Courbin et al. 2022; Siegal et al. 2022). A further reason facilitating the very shallow water occupation may be the long-term protection of turtles in Diego Garcia, with hawksbill turtles being fully protected since 1970 (Mortimer et al. 2020).
Despite the length of the shallow dives, the short post-dive surface intervals suggest these dives were aerobic, since long surface intervals are required to remove lactate build up from anaerobic metabolism (Costa et al. 2004). Anaerobic metabolism in diving sea turtles seems to occur mainly under duress, for example in forced experimental submergences (Lutz and Bentley 1985) or entanglement in fishing nets (Snoddy and Southwood Williard 2010; Miguel et al. 2020). Our conclusion of routine aerobic diving supports this consensus view that sea turtles, in line with other air-breathing divers including mammals and birds, are usually diving aerobically (Southwood Williard 2013). Aerobic diving leads to higher overall dive efficiency as it negates the need for long surface intervals and is a far more efficient form of energy conversion, with each glucose molecule creating 19 times more ATP via aerobic pathways than anaerobically (Southwood Williard 2013).
One reason for long aerobic dives at shallow depths can be because of exceptionally cold water, since metabolic rate reduces at lower temperatures and hence aerobic dive limit increases (e.g. Hochscheid et al. 2004; Luschi et al. 2013). However, this was not the case in the current study since turtles experienced very high temperatures. For example, in a review of water temperatures at breeding sites, temperatures ranging from 22 to 28 °C were described (Hays et al. 2002b), compared to ambient temperatures exceeding 30 °C recorded here for shallow diving hawksbill turtles. As noted by others (Hochscheid et al. 2004; Madrak et al. 2022), we have shown that dive duration increased at lower temperatures, but even at warm temperatures of around 32 °C, nocturnal dives were still around 20 min. Our findings therefore suggest that at shallow foraging sites that are cooler, hawksbill turtles will routinely dive for even longer than we recorded, i.e. long dives in shallow water can likely occur broadly in this species.
While the high variability of sea turtle metabolic rate with temperature (Southwood et al. 2006), activity levels (Williams et al. 2019) and digestive status (Jones et al. 2009) complicates comparisons of dive durations across studies and species, nevertheless, when combined with other dive data from hawksbill turtles, our findings highlight that this species consistently dives for longer than green and loggerhead turtles when depth and temperature are accounted for. The reasons for these long, shallow dives are enigmatic. At least three hypotheses can be suggested: (a) that hawksbills have lower mass-specific metabolic rates than green and loggerhead turtles, (b) they have larger mass-specific oxygen stores or (c) they have greater gas-free weight in water (for example due to lower fat stores), so that a larger lung volume will still attain neutral buoyancy in shallow water. Teasing apart these various alternatives is not straightforward. Measurements of lung volumes suggest hawksbills are not unusual in this regard (Hochscheid et al. 2007). There are no data on gas-free weights in water for sea turtles, i.e. the animal weight that the lungs need to support which will drive lung volume in attaining neutral buoyancy. Likewise there are no measurements of relative fat stores across species as yet. Recent evidence has shown differences in mass-specific blood volumes and hence oxygen stores across cheloniid turtles, showing that species may have different adaptations for diving (Arango et al. 2021) although data for hawksbill turtles are lacking.
Our cross taxa comparisons revealed high dive efficiency in hawksbill turtles, comparable to other air-breathing marine divers. Metrics of dive efficiency will be influenced by the threshold used to define depths. For example, when looking at the % time submerged, a deeper dive threshold will lead to calculations of lower dive efficiency. So our calculated high % of time submerged will be due, at least partly, to our shallow threshold (0.2 m) to define a dive and this metric will not be strictly comparable to other divers where this threshold is typically much deeper (2–5 m; Weise et al. 2010; Zimmer et al. 2010). Nevertheless, even when we looked at the % of the dive in the bottom phase, hawksbills still had high dive efficiency compared to other divers. Deeper divers will clearly need to spend longer in transit from the surface to the bottom phase of their dives, which will lower their dive efficiency compared to hawksbills. Regardless of the caveats of these dive efficiency metrics, it is clear that hawksbills can spend a high proportion of their time submerged, even when diving in very shallow water.
There are several reasons why cheloniid turtles, in general, might show high dive efficiency compared to marine mammals and birds. Cheloniid turtles tend to dive to shallower depths than many marine mammals and hence energy expenditure during descent and ascent is likely reduced; they tend to feed on sessile invertebrates or plants and hence energy expenditure in prey pursuit is minimised; unlike endotherms, they do not expend energy warming cold prey in their stomachs; being ectothermic, their rates of oxygen consumption are considerably lower (Hayward et al. 2016). In contrast, leatherback turtles (Dermochelys coriacea) have lower dive efficiency and tend to be regionally endothermic, dive deeper and re-warm cold prey in their stomachs (Casey et al. 2010), making them more akin to diving birds and marine mammals. Optimisation of energetic gains can be achieved by either reducing the metabolic cost of foraging or maximising energy intake, and predators of mobile prey are known to switch between these strategies based on prey patch density in order to achieve maximal overall energy efficiency (e.g. blue whales, Balaenoptera musculus, Hazen et al. 2015). Hawksbill turtles and other benthic grazers employing lower cost feeding mechanisms are likely to minimise the metabolic cost of foraging dives by maximising dive efficiency, while species in pursuit of active prey may finish a dive before oxygen stores are depleted if prey are not encountered (Thums et al. 2013), or are likely to have lower dive efficiency due to the higher energetic demands of catching mobile prey.
In conclusion, while generally across studies with marine mammals and birds the focus has been on diving to 10s of metres or more (Williams and Ponganis 2021), our work highlights that shallow water habitats may be used very efficiently by air-breathing divers, both to forage and also likely reduce predation risk, highlighting the need for further consideration of shallow water diving across other diving species.
Data availability
The data described in this article are publicly available from the Dryad Digital Repository (https://doi.org/10.5061/dryad.c2fqz61c1).
References
Arango B, Harfush-Meléndez M, Marmolejo-Valencia JA, Merchant-Larios H, Crocker DE (2021) Blood oxygen stores of olive ridley sea turtles, Lepidochelys olivacea are highly variable among individuals during arribada nesting. J Comp Physiol B 191:185–194. https://doi.org/10.1007/s00360-020-01321-1
Ballorain K, Bourjea J, Ciccione S, Kato A, Hanuise N, Enstipp M, Fossette S, Georges J-Y (2013) Seasonal diving behaviour and feeding rhythms of green turtles at Mayotte Island. Mar Ecol Prog Ser 483:289–302. https://doi.org/10.3354/meps10301
Blumenthal JM, Austin TJ, Bothwell JB, Broderick AC, Ebanks-Petrie G, Olynik JR, Orr MF, Solomon JL, Witt MJ, Godley BJ (2009) Diving behaviour and movements of juvenile hawksbill turtles Eretmochelys imbricata on a Caribbean coral reef. Coral Reefs 28:55–65. https://doi.org/10.1007/s00338-008-0416-1
Brill RW, Balazs GH, Holland KN, Chang RKC, Sullivan S, George JC (1995) Daily movements, habitat use, and submergence intervals of normal and tumor-bearing juvenile green turtles (Chelonia mydas L.) within a foraging area in the Hawaiian islands. J Exp Mar Biol Ecol 185:203–218. https://doi.org/10.1016/0022-0981(94)00146-5
Casey J, Garner J, Garner S, Southwood Williard A (2010) Diel foraging behaviour of gravid leatherback sea turtles in deep waters of the Caribbean Sea. J Exp Biol 213:3961–3971. https://doi.org/10.1242/jeb.048611
Cerritelli G, Casale P, Sözbilen D, Hochscheid S, Luschi P, Kaska Y (2022) Multidirectional migrations from a major nesting area in Turkey support the widespread distribution of foraging sites for loggerhead turtles in the Mediterranean. Mar Ecol Prog Ser 683:169–177. https://doi.org/10.3354/meps13946
Chambault P, de Thoisy B, Kelle L, Berzins R, Bonola M, Delvaux H, Le Maho Y, Chevallier D (2016) Inter-nesting behavioural adjustments of green turtles to an estuarine habitat in French Guiana. Mar Ecol Prog Ser 555:235–248. https://doi.org/10.3354/meps11813
Chilvers BL, Delean S, Gales NJ, Holley D, Lawler IR, Marsh H, Preen AR (2004) Diving behaviour of dugongs, Dugong dugon. J Exp Mar Biol Ecol 304:203–224. https://doi.org/10.1016/j.jembe.2003.12.010
Churchward CA (2001) The effect of depth and activity type on dugong (Dugong dugon) diving behaviour in Shark Bay, Western Australia. MSc thesis, University of Calgary, Alberta, Canada. https://doi.org/10.11575/PRISM/16057
Costa DP, Kuhn CE, Weise MJ, Shaffer SA, Arnould JPY (2004) When does physiology limit the foraging behaviour of freely diving mammals? Int Congr Ser 1275:359–366. https://doi.org/10.1016/j.ics.2004.08.058
Courbin N, Pichegru L, Seakamela M, Makhado A, Meÿer M, Kotze PGH, Mc Cue SA, Péron C, Grémillet D (2022) Seascapes of fear and competition shape regional seabird movement ecology. Comm Biol 5:208. https://doi.org/10.1038/s42003-022-03151-z
Dunn N, Savolainen V, Weber S, Andrzejackzek S, Carbone C, Curnick D (2022) Elasmobranch diversity across a remote coral reef atoll revealed through environmental DNA metabarcoding. Zool J Linn Soc. https://doi.org/10.1093/zoolinnean/zlac014
Fahlman A, Moore MJ, Garcia-Parraga D (2017) Respiratory function and mechanics in pinnipeds and cetaceans. J Exp Biol 220:1761–1773. https://doi.org/10.1242/jeb.126870
Gradcylinder.org (2022) The graduated cylinder: fit quadratic-plateau models in R. https://gradcylinder.org/quad-plateau/. Accessed Feb 2022
Grémillet D, Argentin G, Schulte B, Culik BM (1998) Flexible foraging techniques in breeding cormorants Phalacrocorax carbo and shags Phalacrocorax aristotelis: benthic or pelagic feeding? Ibis 140:113–119. https://doi.org/10.1111/j.1474-919X.1998.tb04547.x
Hamylton S, East H (2012) A geospatial appraisal of ecological and geomorphic change on Diego Garcia atoll, Chagos Islands (British Indian Overseas Territory). Remote Sens 4:34444–43461. https://doi.org/10.3390/rs4113444
Harrington LA, Hays GC, Fasola L, Harrington AL, Righton D, Macdonald DW (2012) Dive performance in a small-bodied, semi-aquatic mammal in the wild. J Mammal 93:198–210. https://doi.org/10.1644/10-MAMM-A-351.1
Hays GC, Broderick AC, Glen F, Godley BJ, Houghton JDR, Metcalfe JD (2002a) Water temperature and internesting intervals for loggerhead (Caretta caretta) and green (Chelonia mydas) sea turtles. J Therm Biol 27:429–432. https://doi.org/10.1016/S0306-4565(02)00012-8
Hays GC, Glen F, Broderick AC, Godley BJ, Metcalfe JD (2002b) Behavioural plasticity in a large marine herbivore: contrasting patterns of depth utilisation between two green turtle (Chelonia mydas) populations. Mar Biol 141:985–990. https://doi.org/10.1007/s00227-002-0885-7
Hays GC, Metcalfe JD, Walne AW (2004) The implications of lung regulated buoyancy control for dive depth and duration. Ecology 85:1137–1145. https://doi.org/10.1890/03-0251
Hays GC, Forman DW, Harrington LA, Harrington AL, Macdonald DW, Righton D (2007) Recording the free-living behaviour of small-bodied, shallow-diving animals with data loggers. J Anim Ecol 76:183–190. https://doi.org/10.1111/j.1365-2656.2006.01181.x
Hays CG, Mortimer JA, Rattray A, Shimada T, Esteban N (2021) High accuracy tracking reveals how small conservation areas can protect marine megafauna. Ecol App 31:e02418. https://doi.org/10.1002/eap.2418
Hayward A, Pajuelo M, Haase CG, Anderson DM, Gillooly JF (2016) Common metabolic constraints on dive duration in endothermic and ectothermic vertebrates. PeerJ 4:e2569. https://doi.org/10.7717/peerj.2569
Hazel J, Lawler IR, Hamann M (2009) Diving at the shallow end: green turtle behaviour in near-shore foraging habitat. J Exp Mar Biol Ecol 371:84–92. https://doi.org/10.1016/j.jembe.2009.01.007
Hazen EL, Friedlaender AS, Goldbogen JA (2015) Blue whales (Balaenoptera musculus) optimise foraging efficiency by balancing oxygen use and energy gain as a function of prey density. Sci Adv 1:e1500469. https://doi.org/10.1126/sciadv.1500469
Heithaus MR, Frid A (2003) Optimal diving under the risk of predation. J Theor Biol 223:79–92. https://doi.org/10.1016/S0022-5193(03)00073-0
Heithaus MR, Frid A, Dill LM (2002) Shark-inflicted injury frequencies, escape ability, and habitat use of green and loggerhead turtles. Mar Biol 140:229–236. https://doi.org/10.1007/s00227-001-0712-6
Heithaus MR, Frid A, Wirsing AJ, Dill LM, Fourqurean JW, Burkholder D, Thomson J, Bejder L (2007) State-dependent risk-taking by green sea turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. J Anim Ecol 76:837–844. https://doi.org/10.1111/j.1365-2656.2007.01260.x
Heithaus MR, Wirsing AJ, Thomson JA, Burkholder DA (2008) A review of lethal and non-lethal effects of predators on adult marine turtles. J Exp Mar Biol Ecol 356:43–51. https://doi.org/10.1016/j.jembe.2007.12.013
Hill JE, Robinson NJ, King CM, Paladino FV (2017) Diving behavior and thermal habitats of gravid hawksbill turtles at St. Croix, USA. Mar Biol 164:17. https://doi.org/10.1007/s00227-016-3050-4
Hochscheid S (2014) Why we mind sea turtles’ underwater business: a review on the study of diving behaviour. J Exp Mar Biol Ecol 450:118–136. https://doi.org/10.1016/j.jembe.2013.10.016
Hochscheid S, Godley BJ, Broderick AC, Wilson RP (1999) Reptilian diving: highly variable dive patterns in the green turtle Chelonia mydas. Mar Ecol Prog Ser 185:101–112. https://doi.org/10.3354/meps185101
Hochscheid S, Bentivegna F, Speakman JR (2003) The dual function of the lung in chelonian sea turtles: buoyancy control and oxygen storage. J Exp Mar Biol Ecol 297:123–140. https://doi.org/10.1016/j.jembe.2003.07.004
Hochscheid S, Bentivegna F, Speakman JR (2004) Long-term cold acclimation leads to high Q10 effects on oxygen consumption of loggerhead sea turtles Caretta caretta. Physiol Biochem Zool 77:209–222. https://doi.org/10.1086/381472
Hochscheid S, McMahon CR, Bradshaw CJA, Maffucci F, Bentivegna F, Hays GC (2007) Allometric scaling of lung volume and its consequences for marine turtle diving performance. Comp Biochem Physiol 148:360–367. https://doi.org/10.1016/j.cbpa.2007.05.010
Houghton JDR, Woolmer A, Hays GC (2000) Sea turtle diving and foraging behaviour around the Greek island of Kefalonia. J Mar Biol Assoc UK 80:761–762. https://doi.org/10.1017/S002531540000271x
Houghton JDR, Broderick AC, Godley BJ, Metcalfe JD, Hays GC (2002) Diving behaviour during the internesting interval for loggerhead turtles Caretta caretta nesting in Cyprus. Mar Ecol Prog Ser 227:63–70. https://doi.org/10.3354/meps227063
Houghton JDR, Callow MJ, Hays GC (2003) Habitat utilisation by juvenile hawksbill turtles (Eretmochelys imbricata, Linnaeus, 1766) around a shallow water coral reef. J Nat Hist 37:1269–1280. https://doi.org/10.1080/00222930110104276
Houghton JDR, Doyle TK, Davenport J, Wilson RP, Hays GC (2008) The role of infrequent and extraordinary deep dives in leatherback turtles (Dermochelys coriacea). J Exp Biol 211:2566–2575. https://doi.org/10.1242/jeb.020065
Jones TT, Hastings MD, Bostrom BL, Andrews RD, Jones DR (2009) Validation of the use of doubly labeled water for estimating metabolic rate in the green turtle (Chelonia mydas L.): a word of caution. J Exp Biol 212:2635–2644. https://doi.org/10.1242/jeb.029330
Luschi P, Mencacci R, Vallini C, Ligas A, Lombardi P, Benvenuti S (2013) Long-term tracking of adult loggerhead turtles (Caretta caretta) in the Mediterranean Sea. J Herpetol 47:227–231. https://doi.org/10.1670/11-173
Lutcavage ME, Lutz PL (1997) Diving physiology. In: Lutz PL, Musick JA (eds) The biology of sea turtles, vol 1. CRC Press, Boca Raton, pp 297–314
Lutz PL, Bentley TB (1985) Respiratory physiology of diving in the sea turtle. Copeia 1985:671–679. https://doi.org/10.2307/1444761
Madrak SV, Lewison RL, Eguchi T, Klimley AP, Seminoff JA (2022) Effects of ambient temperature on dive behavior of East Pacific green turtles before and after a power plant closure. Mar Ecol Prog Ser 683:157–168. https://doi.org/10.3354/meps13940
Makowski C, Seminoff JA, Salmon M (2006) Home range and habitat use of juvenile Atlantic green turtles (Chelonia mydas L.) on shallow reef habitats in Palm Beach, Florida, USA. Mar Biol 148:1167–1179. https://doi.org/10.1007/s00227-005-0150-y
Matley JK, Jossart J, Johansen L, Jobsis PD (2020) Environmental drivers of diving behaviour and space-use of juvenile endangered Caribbean hawksbill sea turtles identified using acoustic telemetry. Mar Ecol Prog Ser 652:157–171. https://doi.org/10.3354/meps13466
McDonald BI, Ponganis PJ (2012) Lung collapse in the diving sea lion: hold the nitrogen and save the oxygen. Biol Lett 8:1047–1049. https://doi.org/10.1098/rsbl.2012.0743
Miguel C, Becker JH, de Freitas BS, Touguinha LBA, Salvador M, Oliveira GT (2020) Physiological effects of incidental capture and seasonality on juvenile green sea turtles (Chelonia mydas). J Exp Mar Biol Ecol 533:151460. https://doi.org/10.1016/j.jembe.2020.151460
Mortimer JA, Esteban N, Guzman AN, Hays GC (2020) Estimates of marine turtle nesting populations in the south-west Indian Ocean indicate the importance of the Chagos Archipelago. Oryx 54:332–343. https://doi.org/10.1017/S0030605319001108
Peterson CC, Gomez D (2008) Buoyancy regulation in two species of freshwater turtle. Herpetologica 64:141–148. https://doi.org/10.1655/07-050.1
Ponganis PJ (2011) Diving mammals. Comp Physiol 1:447–465. https://doi.org/10.1002/cphy.c091003
Ponganis PJ, Kooyman GL (2000) Diving physiology of birds: a history of studies on polar species. Comp Biochem Physiol A 126:143–151. https://doi.org/10.1016/S1095-6433(00)00208-7
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.s://www.R-project.org
Sato K, Naito Y, Kato A, Niizuma Y, Watanuki Y, Charrassin JB, Bost CA, Handrich Y, Le Maho Y (2002) Buoyancy and maximal diving depth in penguins: do they control inhaling air volume? J Exp Biol 205:1189–1197. https://doi.org/10.1242/jeb.205.9.1189
Schagatay E, Lodin-Sundström A, Abrahamsson E (2011) Underwater working times in two groups of traditional apnea divers in Asia: the Ama and the Bajau. Diving Hyperb Med 41:27–30
Schofield G, Bishop CM, Katselidis KA, Dimopoulos P, Pantis JD, Hays GC (2009) Microhabitat selection by sea turtles in a dynamic thermal marine environment. J Anim Ecol 78:14–21. https://doi.org/10.1111/j.1365-2656.2008.01454.x
Seminoff JA, Jones TT, Marshall GJ (2006) Underwater behaviour of green turtles monitored with video-time-depth recorders: what’s missing from dive profiles? Mar Ecol Prog Ser 322:269–280. https://doi.org/10.3354/meps322269
Siegal E, Hooker SK, Isojunno S, Miller PJO (2022) Beaked whales and state-dependent decision-making: how does body condition affect the trade-off between foraging and predator avoidance? Proc Royal Soc B 289:20212539. https://doi.org/10.1098/rspb.2021.2539
Sims DW, Wearmouth VJ, Southall EJ, Hill JM, Moore P, Rawlinson K, Hutchinson N, Budd GC, Righton D, Metcalfe JD, Nash JP, Morritt D (2005) Hunt warm, rest cool: bioenergetic strategy underlying diel vertical migration of a benthic shark. J Anim Ecol 75:176–190. https://doi.org/10.1111/j.1365-2656.2005.01033.x
Snoddy JE, Southwood Williard A (2010) Movements and post-release mortality of juvenile sea turtles released from gillnets in the lower Cape Fear River, North Carolina, USA. Endanger Species Res 12:235–247. https://doi.org/10.3354/esr00305
Stokes KL, Esteban N, Stokes HJ, Hays GC (2023) Data from: High dive efficiency in shallow water. Dryad Digital Repository. https://doi.org/10.5061/dryad.c2fqz61c1
Southwood AL, Reina RD, Jones VS, Speakman JR, Jones DR (2006) Seasonal metabolism of juvenile green turtles (Chelonia mydas) at Heron Island, Australia. Can J Zool 84:125–135. https://doi.org/10.1139/Z05-185
Southwood Williard A (2013) Physiology as integrated systems. In: Wyneken J, Lohmann KJ, Musick JA (eds) The biology of sea turtles, vol III. CRC Press, Boca Raton, pp 1–30
Teo SLH, Boustany A, Dewar H, Stokesbury MJW, Weng KC, Beemer S, Seitz AC, Farwell CJ, Prince ED, Block BA (2007) Annual migrations, diving behaviour, and thermal biology of Atlantic bluefin tuna, Thunnus thynnus, on their Gulf of Mexico breeding grounds. Mar Biol 151:1–18. https://doi.org/10.1007/s00227-006-0447-5
Thomson JA, Heithaus MR, Dill LM (2011) Informing the interpretation of dive profiles using animal-borne video: A marine turtle case study. J Exp Mar Biol Ecol 410:12–20. https://doi.org/10.1016/j.jembe.2011.10.002
Thomson JA, Cooper AB, Burkholder DA, Heithaus MR, Dill LM (2012) Heterogeneous patterns of availability for detection during visual surveys: spatiotemporal variation in sea turtle diving—surfacing behaviour on a feeding ground. Methods Ecol Evol 3:378–387. https://doi.org/10.1111/j.2041-210X.2011.00163.x
Thums M, Bradshaw CJA, Sumner MD, Horsburgh JM, Hindell MA (2013) Depletion of deep marine food patches forces divers to give up early. J Anim Ecol 82:72–83. https://doi.org/10.1111/j.1365-2656.2012.02021.x
van Dam RP, Diez CE (1997) Diving behaviour of immature hawksbills (Eretmochelys imbricata) in a Caribbean reef habitat. Coral Reefs 16:133–138. https://doi.org/10.1007/s003380050067
Watwood SL, Miller PJO, Johnson M, Madsen PT, Tyack PL (2006) Deep-diving foraging behaviour of sperm whales (Physeter macrocephalus). J Anim Ecol 75:814–825. https://doi.org/10.1111/j.1365-2656.2006.01101.x
Weise MJ, Harvey JT, Costa DP (2010) The role of body size in individual-based foraging strategies of a top marine predator. Ecology 91(4):1004–1015. https://doi.org/10.1890/08-1554.1
Williams CL, Ponganis PJ (2021) Diving physiology of marine mammals and birds: the development of biologging techniques. Philos Trans R Soc Lond B Biol Sci 376:20200211. https://doi.org/10.1098/rstb.2020.0211
Williams CL, Sato K, Ponganis PJ (2019) Activity not submergence explains diving heart rates of captive loggerhead turtles. J Exp Biol 222:jeb200824. https://doi.org/10.1242/jeb.200824
Wilson RP (2003) Penguins predict their performance. Mar Ecol Prog Ser 249:305–310. https://doi.org/10.3354/meps249305
Winterbottom R, Anderson RC (1997) A revised checklist of the epipelagic and shore fishes of the Chagos Archipelago, Central Indian Ocean. JLB Smith Inst Ichthyol 66:1–28
Witt MJ, McGowan A, Blumenthal JM, Broderick AC, Gore S, Wheatley D, White J, Godley BJ (2010) Inferring vertical and horizontal movements of juvenile marine turtles from time-depth recorders. Aquat Biol 8:169–177. https://doi.org/10.3354/ab00221
Zimmer I, Wilson RP, Beaulieu M, Ropert-Coudert Y, Kato A, Ancel A, Plötz J (2010) Dive efficiency versus depth in foraging emperor penguins. Aquat Biol 8:269–277. https://doi.org/10.3354/ab00213
Additional data sources
Andrews RD, Costa DP, Boeuf Le BJ, Jones DR (2000) Breathing frequencies of northern elephant seals at sea and on land revealed by heart rate spectral analysis. Resp Physiol 123:71–85. https://doi.org/10.1016/S0034-5687(00)00168-7
Balazs G (1994) Homeward bound:satellite tracking of Hawaiian green turtles from nesting beaches to foraging pastures. In: Proceedings of the thirteenth annual symposium on sea turtle biology and conservation (ed. BA Schroeder, BE Witherington). NOAA Technical Memorandum NMFS-SEFC-341, United States Department of Commerce, pp 205–208
Bekkby T, Bjørge A (2000) Diving behaviour of harbour seal Phoca vitulina pups from nursing to independent feeding. J Sea Res 44:267–275. https://doi.org/10.1016/S1385-1101(00)00048-4
Bell IP, Parmenter CJ (2008) The diving behaviour of inter-nesting hawksbill turtles, Eretmochelys imbricata (Linnaeus 1766), on Milman Island reef, Queensland, Australia. Herpetol Conserv Biol 3:254–263
Blumenthal JM, Austin TJ, Bothwell JB, Broderick AC, Ebanks-Petrie G, Olynik JR, Orr MF, Solomon JL, Witt MJ, Godley BJ (2010) Life in (and out of) the lagoon: fine-scale movements of green turtles tracked using time-depth recorders. Aquat Biol 9:113–121. https://doi.org/10.3354/ab00222
Born EW, Rysgaard S, Ehlmé G, Sejr M, Acquarone M, Levermann N (2003) Underwater observations of foraging in free-living Atlantic walruses (Odobenus rosmarus rosmarus) and estimates of their food consumption. Polar Biol 26:348–357. https://doi.org/10.1007/s00300-003-0486-z
Brischoux F, Bonnet X, Cook T, Shine R (2007) Snakes at sea: diving performance of free-ranging sea kraits. In: 11th Annual Meeting on Health, Science and Technology. Université François Rabelais, Tours, France, pp 5–20
Butler PJ, Jones DR (1997) Physiology of diving of birds and mammals. Physiol Rev 77:837–899. https://doi.org/10.1152/physrev.1997.77.3.837
Cheng IJ, Bentivegna F, Hochscheid S (2013) The behavioural choices of green turtles nesting at two environmentally different islands in Taiwan. J Exp Mar Biol Ecol 440:141–148. https://doi.org/10.1016/j.jembe.2012.12.002
Cook TR, Brischoux F (2014) Why does the only ‘planktonic tetrapod’ dive? Determinants of diving behaviour in a marine ectotherm. Anim Behav 98:113–123. https://doi.org/10.1016/j.anbehav.2014.09.018
Costa DP, Gales NJ (2000) Foraging energetics and diving behaviour of lactating New Zealand sea lions, Phocarctos hookeri. J Exp Biol 203:3655–3665
Folkow LP, Blix AS (1999) Diving behaviour of hooded seals (Cystophora cristata) in the Greenland and Norwegian Seas. Polar Biol 22:61–74. https://doi.org/10.1007/BF02329206
Hays GC, Adams CR, Broderick AC, Godley BJ, Lucas DJ, Metcalfe JD, Prior AA (2000) The diving behaviour of green turtles at Ascension Island. Anim Behav 59:577–586. https://doi.org/10.1006/anbe.1999.1326
Houghton JDR, Cedras A, Myers AE, Liebsch N, Metcalfe JD, Mortimer JA, Hays GC (2008) Measuring the state of consciousness in a free-living diving sea turtle. J Exp Mar Biol Ecol 356:115–120. https://doi.org/10.1016/j.jembe.2007.12.008
Iverson AR, Fujisaki I, Lamont MM, Hart KM (2019) Loggerhead sea turtle (Caretta caretta) diving changes with productivity, behavioural mode, and sea surface temperature. PLoS One 14:e0220372. https://doi.org/10.1371/journal.pone.0220372
Mate BR, Rossbach KA, Nieukirk SL, Wells RS, Irvine AB, Scott MD, Read AJ (1995) Satellite-monitored movements and dive behaviour of a bottlenose dolphin (Tursiops truncatus) in Tampa Bay, Florida. Mar Mamm Sci 11:452–463. https://doi.org/10.1111/j.1748-7692.1995.tb00669.x
Minamikawa S, Naito Y, Uchida I (1997) Buoyancy control and diving behaviour of the loggerhead turtle, Caretta caretta. J Ethol 15:109–118. https://doi.org/10.1007/BF02769396
Okuyama J, Kataoka K, Kobayashi M, Abe O, Yoseda K, Arai N (2012) The regularity of dive performance in sea turtles: a new perspective from precise activity data. Anim Behav 84:349–359. https://doi.org/10.1016/j.anbehav.2012.04.033
Page B, McKenzie J, Goldsworthy SD (2005) Inter-sexual differences in New Zealand fur seal diving behaviour. Mar Ecol Prog Ser 304:249–264. https://doi.org/10.3354/meps304249
Pütz K, Wilson RP, Charrassin J-B, Raclot T, Lage J, Maho Le Y, Kierspel MAM, Culik BM, Adelung D (1998) Foraging strategy of king penguins (Aptenodytes patagonicus) during summer at the Crozet Islands. Ecology 79:1905–1921. https://doi.org/10.2307/176698
Renauld ML, Williams JA (2005) Kemp’s ridley sea turtle movements and migrations. Chelonian Conserv Biol 4:808–816
Sakamoto W, Sato K, Tanaka H, Naito Y (1993) Diving patterns and swimming environment of two loggerhead turtles during internesting. Nippon Suisan Gakkaishi 59:1129–1137. https://doi.org/10.2331/suisan.59.1129
Schreer JF, Kovacs KM, O’Hara Hines RJ (2001) Comparative diving patterns of pinnipeds and seabirds. Ecol Monogr 71:137–162. https://doi.org/10.1890/0012-9615(2001)071[0137:cdpopa]2.0.co;2
Shero MR, Goetz KT, Costa DP, Burns JM (2018) Temporal changes in Weddell seal dive behavior over winter: Are females increasing foraging effort to support gestation? Ecol Evol 8:11857–11874. https://doi.org/10.1002/ece3.4643
Slip DJ, Hindell MA, Burton HR (1994) Diving behaviour of southern elephant seals from Macquarie Island: an overview. In: Boeuf Le BJ, Laws RM (eds) Elephant seals: population ecology, behaviour, and physiology. University of California Press Los Angeles 253:270
Southwood AL, Andrews RD, Lutcavage ME, Paladino FV, West NH, George RH, Jones DR (1999) Heart rates and diving behaviour of leatherback sea turtles in the Eastern Pacific ocean. J Exp Biol 202:1115–1125. https://doi.org/10.1242/jeb.202.9.1115
Storch S, Hays GC, Hillis-Starr Z, Wilson RP (2006) The behaviour of a hawksbill turtle data-logged during the passage of hurricane Georges through the Caribbean. Mar Freshw Behav Physiol 39:307–313. https://doi.org/10.1080/10236240600919796
Thompson D, Fedak MA (1993) Cardiac responses of grey seals during diving at sea. J Exp Biol 174:139–164. https://doi.org/10.1242/jeb.174.1.139
Dam van RP, Diez CE (1996) Diving behaviour of immature hawksbills (Eretmochelys imbricata) in a Caribbean cliff-wall habitat. Mar Biol 127:171–178. https://doi.org/10.1007/BF00993657
Walcott J, Eckert S, Horrocks JA (2013) Diving behaviour of hawksbill turtles during the inter-nesting interval: strategies to conserve energy. J Exp Mar Biol Ecol 448:171–178. https://doi.org/10.1016/j.jembe.2013.07.007
Wright JC (1987) Energy metabolism during unrestrained submergence in the saltwater crocodile Crocodylus porosus. Physiol Zool 60:515–523. https://doi.org/10.1086/physzool.60.5.30156126
Yasuda T, Arai N (2009) Changes in flipper beat frequency, body angle and swimming speed of female green turtles Chelonia mydas. Mar Ecol Prog Ser 386:275–286. https://doi.org/10.3354/meps08084
Acknowledgements
We thank two anonymous reviewers for comments which helped to improve the manuscript.
Funding
This work was supported by the Bertarelli Foundation as part of the Bertarelli Programme in Marine Science (projects 2017-4, 820633).
Author information
Authors and Affiliations
Contributions
GCH conceived the manuscript. NE, HJS and GCH conducted the fieldwork. KLS and GCH led the data analysis and writing with contributions from all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing or financial interests.
Ethical approval
This study was approved by Swansea University and Deakin University Ethics Committees and the British Indian Ocean Territory Administration (BIOTA) of the UK Foreign, Commonwealth and Development Office. The study was endorsed through research permits (0002SE12, 0002SE14, 0007SE15, 0002SE17, 0006SE18, 0009SE18, 0004SE19, 0011SE19, 0001SE21) from the Commissioner’s Representative for BIOT, and research complied with all relevant local and national legislation.
Additional information
Responsible Editor: D. Crocker.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stokes, K.L., Esteban, N., Stokes, H.J. et al. High dive efficiency in shallow water. Mar Biol 170, 45 (2023). https://doi.org/10.1007/s00227-023-04179-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04179-3