Abstract
Understanding large-scale spatial and temporal patterns of marine populations is a central goal in ecology, which has received renewed attention under climate change. However, few studies explore the large-scale dynamics of populations using standardized protocols and during the same time frames. We studied the phenology and intensity of reproduction and recruitment for the intertidal stalked barnacle Pollicipes pollicipes over an European scale and described their potential linkages with environmental variables. This species supports profitable fisheries in the Iberian Peninsula (Spain and Portugal). In Brittany (France), we had observed a significant lower reproductive effort (long non-breeding season, short breeding period in summer) and low values of recruitment intensity. This pattern may be related to the fact that Brittany corresponds to the northern limit of the distribution of this species in continental Europe. On the Iberian Peninsula, the most different region was Galicia (Spain), with Asturias (Spain) and SW Portugal being more similar. In Galicia, we have observed a contradictory pattern characterized by the absence of a non-breeding period and by a shorter recruitment season than observed in other Iberian regions. Our results suggest that air temperature, SST and chlorophyll-a might be related to the variability in reproduction and recruitment patterns of P. pollicipes. Moreover, spring and early summer upwelling in SW Portugal and Galicia might be inhibiting recruitment in this period. At the northern limit, the expected increase in performance under climate change might facilitate the recovery of populations after exploitation, increasing the resilience of the resource to fishing pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding and predicting the spatio-temporal dynamics of marine populations remains a central goal in ecology and has applied relevance for the conservation of marine biodiversity and management of fisheries resources. In recent decades, this interest has also been pushed by large-scale environmental disturbances such as the impacts of climate change (Poloczanska et al. 2016; Fredston et al. 2021).
Across the geographic range of a species, there might be variation of vital rates (e.g., recruitment, survival, growth and reproduction), and range limits might be explained by less adaptation of organisms to changes in environmental conditions, but also by dispersal dynamics, habitat arrangements or demographic effects (see revision of Brown et al. 1996). For many benthic marine invertebrates with sessile adults and dispersive larvae, recruitment is a key demographic process structuring adult populations (e.g., Connell 1985; Gaines and Roughgarden 1985). Recruitment combines settlement and survival to a certain time or life phase (Connell 1985). Many processes affect the recruitment of a species, but the journey to the benthic phase is originally dependent on the reproductive output of that species (see revision of processes in Pineda 2000). Understanding how the starting point (reproduction) and end point (recruitment to benthic phase) of this journey may vary regionally is, therefore, of fundamental ecological importance to explain spatial and temporal patterns in population and community structure.
Phenology (the timing of life cycle events) and intensity of reproduction and recruitment might change across the geographic distribution of a species. Recruitment or repopulation failure has long been considered a cause of geographic range boundaries and distribution gaps in intertidal invertebrates (e.g., Lewis 1986; Gaylord and Gaines 2000; Gilman 2006). However, there are not many studies of large-scale comparisons of phenology and intensity of these processes in nearshore benthic invertebrates (but see Connolly et al. 2001 and Broitman et al. 2008 for recruitment of barnacles and mussels in the northeast Pacific Ocean, from ~ 34º to 46º N).
In the northeast Atlantic Ocean, the intertidal zone has a long history of ecological and biogeographic study (see revision of Hawkins et al. 2019). But despite this knowledge, we are not aware of any study on large-scale patterns of reproduction and recruitment that has crossed the continental (Europe/Africa) border. In addition, just a few studies have studied these processes on a large-scale in Europe using standardized protocols and during a same period (but see O’Riordan et al. 2004 for recruitment of barnacles; Philippart et al. 2012 for larval occurrence). Regional variation of recruitment of the warm-temperate intertidal barnacle Chthamalus montagui (northern limit in Britain, southern limit in NW Africa) was detected, evidenced by a lower recruitment intensity of northern populations (SW Ireland) and a longer recruitment season in the south (SW Portugal) (O’Riordan et al. 2004). Also for this species, there are observations from the 1980s indicating that the breeding season in the north (Scotland, Lewis 1986) is shorter than in the south (Mediterranean, Crisp et al. 1981) and that are frequent recruitment failures in the north (Scotland, Lewis 1986). For cold-temperate species (e.g., Patella vulgata, northern limit in Norway, southern limit in Portugal; Semibalanus balanoides, northern limit in Norway, southern limit in NW Spain), there is evidence of a decrease in reproduction success in southern limits (Moore et al. 2011; Herrera et al. 2019).
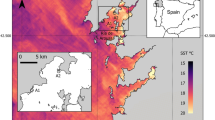
The warm-temperate stalked barnacle Pollicipes pollicipes is distributed in Western Europe and in the North Atlantic African coasts from Brittany (France) to Senegal, being rare in the Mediterranean (Barnes 1996). Northern limit in continental Europe is Plateau de la Méloine (48°43′45′′ N–03°47′14′′ W; E. Thiébaut personal communication), although scattered individuals have been observed in Lands End, SW Britain (50°04′ N) (Southward 2008; Hawkins et al. personal observations) (see Fig. 1a). Along its geographical distribution, there are important stretches of sandy coast that fragment its distribution on the rocky coast, as occurs on the west coast of France south of Brittany and on the NW coast of Africa (see Fig. 1a). This species is abundant on very exposed rocky shores, ranging from the shallow subtidal to mid-intertidal (Barnes 1996) and is heavily exploited in the Iberian Peninsula (Molares and Freire 2003; Cruz et al. 2015). Several fisheries of this species have been identified in Europe, including fisheries close to its northern limit (Brittany, France), and in the Iberian Peninsula, namely in Asturias and Galicia (Spain) and SW Portugal (Aguión et al. 2022). In total, the European fisheries of P. pollicipes have an annual economic value of EUR 10 million, with around 500 tons of landings and 2100 fishers (Aguión et al. 2022). In Spain and Portugal, there is a strong market demand for this resource, whereas in Brittany most of catch is exported to Iberian countries (Aguión et al. 2022).
a Distribution of the stalked barnacle Pollicipes pollicipes (dark grey contour of the coast, based in Cruz et al. accepted) with indication of the four regions studied (Brittany in France, Asturias and Galicia in N Spain, and SW Portugal). Location of the reproduction and recruitment study sites across SW Europe in b Brittany (blue, n = 3), c Galicia in NW Spain (green, n = 6), Asturias in N Spain (orange, n = 6) and d SW Portugal (pink, n = 5). The asterisk in b marks the northernmost observation of the species in continental Europe (Plateau de la Méloine in the NE of the Bay of Morlaix) (E. Thiébaut, personal communication). All sites were used to describe the phenology and intensity of reproduction and recruitment. Map coordinates are in decimal degrees. Projected coordinate system used: WGS 84/Pseudo-Mercator
As most nearshore benthic species, P. pollicipes has a two-phase life cycle, with planktonic larvae (nauplii and cypris) (described in Molares et al. 1994b; Kugele and Yule 1996) and benthic adults. P. pollicipes is a cross-fertilizing simultaneous hermaphrodite and broods its embryos inside the mantle cavity of adults until hatching of stage I nauplii (Cruz et al. 2010). After six planktonic nauplius stages, cyprids of P. pollicipes settle heavily on conspecifics (Barnes 1996). Brooding patterns of this species have been studied by estimating the percentage of adults carrying egg lamellae (e.g., Cardoso and Yule 1995; Cruz and Araújo 1999; Pavón 2003; Macho 2006). Recruitment has been studied by counting cyprids and juveniles attached to the peduncle of adults (De la Hoz and Garcia 1993; Pavón 2003; Cruz et al. 2010; Fernandes et al. 2021) or by determining the percentage of adults with recruits (Molares 1993; Macho 2006). Patterns of reproduction and of recruitment intensity in P. pollicipes in the Iberian Peninsula have been linked with air and sea water temperature, chlorophyll concentration, and upwelling intensity and events (Cardoso and Yule 1995; Cruz and Hawkins 1998; Cruz 2000; Macho 2006; Fernandes et al. 2021).
The general question of this study is to investigate if there are regional differences in the phenology and annual intensity of reproduction (including the number of broods) and of recruitment of P. pollicipes across Europe, specifically in populations close to the northern limit of this species (Brittany) and in Iberian populations (Asturias, Galicia and SW Portugal), using standardized protocols. This study will allow us to better understand the potential impacts of climate change across Europe, namely of air temperature rise and sea water warming (Mieszkowska et al. 2006) and coastal upwelling weakening in NW Iberian Peninsula (Sousa et al. 2020), on the reproduction and recruitment of P. pollicipes and its wider implications for the management of this valuable fishery resource.
Methods
Study sites
Along the coast of SW Europe, 20 sites from four regions were chosen to study P. pollicipes reproduction and recruitment: three sites in Brittany (NW France) close to the northern limit of this species in Continental Europe (Fig. 1b), six sites in Asturias (N Spain), six sites in Galicia (NW Spain) (Fig. 1c), and five sites in SW Portugal (Fig. 1d). All sites correspond to very exposed shores. Two main factors were considered in the selection of the sampling sites: ease of access to maximize visits throughout the year, since P. pollicipes lives in very exposed rocky shores; and to be harvested fishing beds. Both factors were important, as several samplings were conducted by fishers, biologists, and surveillance officers (from cofradías—fishers´s guilds—and regional fisheries administration) that worked in collaboration with the scientific team.
Biological sampling
Monthly collections of barnacles took place at each site during spring tides between July 2017 and July 2019, although sampling was not possible in several dates due to rough sea conditions (Table S1 in SSMM). At each site, clumps were randomly collected from the middle of P. pollicipes intertidal distribution range along approximately 20–100 m of coastline and then frozen until further analyses. On each sampling date and site, 40 adult individuals (rostro-carinal distance (RC) > 15 mm, following Cruz and Hawkins 1998) were randomly chosen (hereafter referred as adults) and measured with a caliper (0.1 mm precision).
Reproduction
On each sampling date and site, the presence of egg lamellae (hereinafter eggs) in the mantle cavity and their maturity status (mature or immature) was registered in 40 adults to calculate the percentage of barnacles with eggs and with mature eggs. Egg masses were considered mature when the black naupliar eye was visible in the embryos and/or when hatched larvae were present, and immature when the naupliar eye was red or still not developed (based in Achituv and Barnes 1978). Regional variation of the phenology, and intensity of reproduction and of the number of broods was determined.
First, we have described the phenology of the main reproductive season and of the non-breeding season for each region by calculating the average percentage of animals with eggs in all sites available on each date (number of sites different according to region and date, see Table S1 in SSMM). We defined the main reproductive season as the period corresponding to the dates when an average of ≥ 50% of the barnacles carried eggs, and the non-breeding season as the period corresponding to the dates when the average percentage of barnacles with eggs was less than 5%.
Regional variation of the phenology and intensity of reproduction was analyzed by multivariate analyses. Multivariate data (reproductive structure) consisted of the values of the percentage of barnacles with eggs in each date (variables) for each site within each region. All sites (n = 3, Brittany; n = 6, Asturias; n = 6, Galicia; n = 5, SW Portugal; see Fig. 1) and sampling dates/months (25) were considered, although not all sites or regions were sampled in all months (see Table 1 in SSMM). First, the data were analyzed according to a one factor (region) experimental design using permutational multivariate analysis of variance (PERMANOVA, Anderson 2001). Second, the average of size (RC) per site was included as a covariate in the PERMANOVA to understand if regional variation was caused by size variation. Finally, we included the interaction between size and region (analogous to the test for homogeneity of slopes in a classical ANCOVA design) to test whether the relationship between reproductive structure and size differed among regions. As differences were found among regions, these were examined using pair-wise tests. As a complement to PERMANOVA, we tested the homogeneity of the dispersions among the four regions by PERMDISP (Anderson 2006), to determine whether different dispersions might be contributing to regional differences. All multivariate analyses were done on the basis of a Bray–Curtis similarity matrix calculated from untransformed data. To visualize the multivariate patterns of reproductive structure, non-metric multidimensional scaling (NMDS) was used as an ordination plot. We superimposed vectors representing the Pearson’s correlation coefficient of the percentage of barnacles with eggs in each sampling date with the two MDS axes (only dates with |r|> 0.8 were included) and bubbles corresponding to the relative average size of the barnacles sampled in each site to visualize the effect of size as a covariate. All analyses were performed using PRIMER 7 (Clarke and Gorley 2015) with the PERMANOVA + add-on (Anderson et al. 2008).
The number of broods was calculated for the year 2018 (the only complete year) following the method proposed by Hilgard (1960) modified for using monthly intervals (see SSMM). The calculation is based on the monthly percentage of individuals carrying egg masses (average of all sites on that region) and the embryo development time. Two approaches were used: the traditional one, where a fixed embryo development time of 25 days is assumed (Molares 1993; Cruz and Araújo 1999; Macho 2006), and one where embryo development time is dependent of temperature, based on embryo development time for other three barnacle species at different temperatures (Patel and Crisp 1960). A detailed explanation on how the embryo development time for P. pollicipes depending on temperature was estimated based on the data from Patel and Crisp (1960) is included in the SSMM (see Fig. S1 and Table S2 in SSMM).
Recruitment
Since the final larval stage (cyprid) of P. pollicipes settles heavily on the peduncle of adult conspecifics (Barnes 1996), we used adults as natural sampling substrates. In each site and sampling date, we randomly selected 20 of the 40 adults used for the reproduction study and counted the cyprids plus juveniles with RC < 0.6 mm (recruits) attached to each adult using a stereo microscope. This size was selected because the estimated average size reached by juveniles with a maximum of 1 month after settlement was 0.50 ± 0.38 mm RC in SW Portugal (mean ± SD; Mateus 2017). As laboratory processing was conducted by different teams in each location (i.e., Sorbonne University, Brittany; University of Vigo, Galicia; University of Oviedo, Asturias; and University of Évora, SW Portugal), a previous training was done among scientists from different regions to avoid biases in the identification and counting of cyprids and juveniles in each region.
First, phenology of the main recruitment season and of the non-recruitment season was described for each region by calculating the average of the mean number of recruits per adult in all sites available on each date (number of sites different according to region and date, see Table S1 in SSMM). Main recruitment season was defined as the period corresponding to the dates when the average of the mean number of recruits was higher than three (based in Cruz et al. 2010). Furthermore, non-recruitment season was defined as the period corresponding to the dates when the average of the mean number of recruits was lower than one.
Regional variation of the phenology and intensity of recruitment was analysed by multivariate analyses. Multivariate data (recruitment structure) consisted of the values of the number of recruits (cyprids plus juveniles with RC < 0.6 mm) per barnacle in each date (variables) for each site within each region. All sites (n = 3, Brittany; n = 6, Asturias; n = 6, Galicia; n = 5, SW Portugal; see Fig. 1) and sampling dates/months (25) were considered, although not all sites or regions were sampled in all months (see Table 1 in SSMM). Since the same barnacles of each site were not sampled on different dates, the allocation of each barnacle to a label (one in 20 replicate barnacles) on each date and site was done randomly. Consequently, 20 “barnacles” per site were considered. First, the data were analysed according to a two factors (region, fixed factor, and site, random factor nested in region) experimental design using permutational multivariate analysis of variance (PERMANOVA, Anderson 2001). Second, the average of size (RC) in all dates per barnacle was included as a covariate in the PERMANOVA to understand if regional and site variation was caused by size variation. Finally, we included the interactions between size and region, and between size and site (analogous to the test for homogeneity of slopes in a classical ANCOVA design) to test whether the relationship between recruitment structure and size differed among regions or sites. As differences were found among regions, these were examined using pair-wise tests. As a complement to PERMANOVA, we tested the homogeneity of the dispersions among the four regions by PERMDISP (Anderson 2006), to determine whether different dispersions might be contributing to regional differences. All multivariate analyses were done on the basis of a Bray–Curtis similarity matrix calculated from square-root transformed data, to reduce the dominant contribution of dates with very high recruitment to Bray–Curtis similarities.
To visualize the multivariate patterns of recruitment structure, non-metric multidimensional scaling (NMDS) was used as an ordination plot, by averaging recruitment data per site for each date. We superimposed vectors representing the Pearson’s correlation coefficient of the number of recruits per barnacle in each sampling date (averaged by site) with the two MDS axes (only dates with |r|> 0.7 were included) and bubbles corresponding to the relative average size of the barnacles sampled in each site to visualize the effect of size as a covariate. All analyses were performed using PRIMER 7 (Clarke and Gorley 2015) with the PERMANOVA + add-on (Anderson et al. 2008).
Environmental variables
The regional monthly average of the following environmental variables was retrieved for the study period (July 2017–2019): sea surface temperature (SST, ºC), air temperature (ºC), chlorophyll-a concentration (mg m−3) and upwelling index (m3 s−1 km−1).
SST and chlorophyll-a concentrations were calculated for two locations of each region (Brittany, Asturias, Galicia and SW Portugal) using satellite data (see geographical coordinates of locations in Table S3 in SSMM). The daily chlorophyll-a data come from the Copernicus operational service from satellite observations resulting from data merging of several sensors (Copernicus data identifier OCEANCOLOUR_ATL_CHL_L4_REP_OBSERVATIONS_009_098) and have a 1 km horizontal resolution. The SST information was extracted from the European North West Shelf/Iberia Biscay Irish seas L4 SST reprocessed (Copernicus data identifier SST_ATL_SST_L4_REP_OBSERVATIONS_010_026), with a 0.05º horizontal resolution (approximately 5.5 and 3.7 km in latitude and longitude, respectively). More information about those products is available through the CMEMS Copernicus system (https://marine.copernicus.eu). Air temperature forecast data computed by the Global Forecast System model with 13 km resolution (GFS 13 km; provided by NOAA—National Oceanic and Atmospheric Administration, U.S. Department of Commerce) was compiled from Windguru archives (www.windguru.cz) for two locations in each of the four study regions (see location geographical coordinates in Table S4 in SSMM), encompassing data with a 6 h frequency. The upwelling index was provided by the Instituto Español de Oceanografía (www.indicedeafloramiento.ieo.es), which is calculated using sea level pressure data from the WXMAP atmospheric model provided by FNMOC (the US Navy Fleet Numerical Meteorology and Oceanography Center) for one location in Asturias and SW Portugal, and two in Galicia (Table S5 in SSMM) using data obtained with a 6 h frequency. Positive values of this index indicate upwelling, while negative values indicate downwelling. Calculating an upwelling index on the scale of the Brittany coast is not relevant according to the hydrodynamics in this region.
To compare the temporal variation of the environmental variables and both processes of reproduction and recruitment, a normalization of the average of the percentage of animals with eggs in all sites, and of the average of the mean number of recruits per adult in all sites was conducted by rescaling the values from each region into a range of 0 to 1.
Results
Reproduction
Reproductive phenology differed among regions (Fig. 2). Nevertheless, in all regions and years, the main reproductive season (≥ 50% of barnacles with eggs) included the months of June, July and August.
The timing of the main reproductive season varied among regions, beginning in both years in April in SW Portugal, May or June depending on the year in Asturias and Galicia, and in June in Brittany (Fig. 2). The end of the main reproductive season was in August in both years in SW Portugal, while it was variable depending on the year in Brittany (July or August) and in Asturias and Galicia (September or October).
In Brittany, the non-breeding season (< 5% of barnacles with eggs) was the longest of all regions. In this region, the non-breeding season was longer in 2017/2018 (from September to May) and shorter in 2018/2019 (October–April). In Asturias, the non-breeding season slightly changed in both years (from December to March in 2017/2018; in November, and in January and February in 2018/2019).
Contrarily, in Galicia, a non-breeding season was absent in both years, although low rates of reproductive intensity (between 6 and 13%) were observed from January to April 2018, and January and February 2019. In SW Portugal, a common non-breeding season was observed in both years from November to January.
At the beginning of the main reproductive season of 2018 (the only complete year), a percentage of animals with mature eggs was observed in Brittany (June, 20.4%), in Asturias (May, 12.1%), in Galicia (June, 28.7%) and in SW Portugal (April, 12.5%) (see Figure S2 in SSMM). In Galicia and SW Portugal, even before the start of the main reproductive season, there were barnacles with mature eggs (Galicia in May, 15.4%, SW Portugal in March, 7.5%) (Figure S2 in SSMM).
Reproductive structure (percentage of barnacles with eggs in each date for each site) (Fig. 3) differed significantly among regions without considering or after considering average size of barnacles (RC) per site as covariate (Table S6 in SSMM). Those differences were not attributable to different dispersions among regions, as homogeneity of dispersions among regions was supported (PERMDISP, F = 2.276; P(perm) = 0.127)). The effect of size (RC) was also significant, but the interaction between region and RC was not significant (Table S6 in SSMM, Fig. 3). Results of pair-wise tests from PERMANOVA without size as covariate indicated that all regions differ from each other (Table S6 in SSMM). When we took into account the effect of RC in the variability among regions, Brittany was significantly different from Iberian regions, and Galicia was significantly different from Asturias and SW Portugal, which were similar (Table S6 in SSMM). Differences between Brittany and Iberian regions might be explained by the longest non-breeding season of Brittany comparing with other regions (Figs. 2 and 3). Differences between Galicia and Asturias and SW Portugal might be explained by a higher intensity of reproduction in late summer and early autumn (September and October) and Winter (January) in Galicia (Fig. 3) and by the overall absence of a non-breeding season in Galicia (Fig. 2).
NMDS bubble plot comparing the reproductive structure (percentage of barnacles with eggs in each date for each site) at each of four regions (3 sites in Brittany, 6 sites in Asturias, 6 sites in Galicia, 5 sites in SW Portugal) based on Bray–Curtis similarities of untransformed data. Vectors indicate direction and magnitude of Pearson correlation coefficient between each variable (percentage of barnacles with eggs in each date) and the MDS axes (only variables with |r|> 0.8 were included). The overlapping date labels on the plot are: 4–19 and 5–19 (above); and 9–18 and 9–17 (below). The size of the bubbles indicates the average size (rostro-carinal length, RC) of the barnacles sampled in each site across the study period
The number of broods estimated at a fixed embryo development time (25 days) was lower in Brittany (2.6 ± 0.5 broods per individual per year) than in Asturias (4.5 ± 0.5), Galicia (5.5 ± 0.9) and SW Portugal (4.9 ± 0.8) (Fig. 4). When calculated under an embryo development time dependent on temperature, the number of broods was considerably smaller in all regions although following the same pattern, i.e., lower in Brittany (2.3 ± 0.4) compared to Asturias (3.7 ± 0.4), Galicia and SW Portugal (both 3.7 ± 0.6).
Annual number of broods per individual by region under a constant embryo development time of 25 days following Molares et al. 1994b (light grey) and under a variable one depending on water temperature (dark grey) estimated in this work (see SSMM). The calculation of the total number of broods as its dispersion (error bars) is explained in the SSMM
Recruitment
Recruitment phenology was variable among regions (Fig. 5), namely a shorter main recruitment season (> 3 recruits per barnacle) in Brittany and Galicia than in Asturias and SW Portugal. In Brittany, only in September 2017 the number of recruits was > 3 recruits per barnacle, and none in 2018 (Fig. 5). In Galicia, the main recruitment season was restricted to July in 2017 and to August in 2018. In Asturias, the main recruitment season lasted from July to December in 2017, and was shorter in 2018, lasting from July to October. In SW Portugal, a shorter main recruitment season was observed in 2017 (July to November), while a longer season was observed in the following year: from August 2018 to January 2019. Non-recruitment months (< 1 recruit per barnacle) were very.
Mean (± SE) number of recruits (cyprids plus juveniles RC < 0.6 mm) per adult in Brittany, Asturias, Galicia and SW Portugal from July 2017 to 2019. Dashed lines indicate the presence of 3 and 1 recruits. The number of sites depends on the date and region (see Table S1 in SSMM for more detailed information). *Note that y axis differs among regions
common in Brittany, Asturias and Galicia from January to June, while in SW Portugal, the non-recruitment season was slightly shorter, lasting from February to June (Fig. 5).
Recruitment structure (number of recruits in each date for each barnacle) (Fig. 6, averaged by site) differed significantly among regions and among sites within each region without considering or after considering average size of barnacles (RC) per site as covariate (Table S7 in SSMM). Regional differences might be attributable to centroid differences or to different dispersions among regions, as homogeneity of dispersions among regions was not supported (PERMDISP, F = 95.291; P(perm) = 0.001)). The effect of size (RC) was also significant, but the interactions between region and RC and between site and RC were not significant (Table S7 in SSMM). Results of pair-wise tests from PERMANOVA without size as covariate indicated that all regions differ from each other (Table S7 in SSMM). When we considered the effect of RC in the variability among regions, the pattern changed. Brittany was only significantly different from Asturias and SW Portugal which indicate that size might be explaining the variability between Brittany and Galicia (detected when we did not consider the effect of size). So, differences between Brittany and Galicia might be attributable to differences in the size (RC) of sampled barnacles that was lower in Brittany (Fig. 6), while detected differences between Brittany and Asturias and SW Portugal cannot be attributable to differences in size. In relation to Iberian regions, Galicia was significantly different from Asturias and SW Portugal, which were similar (Table S7 in SSMM). Differences between Galicia and Asturias and SW Portugal might be explained by a lower intensity of recruitment in late summer and early autumn (August, September and October) in Galicia (Fig. 6) and by the overall shorter main recruitment season in Galicia (Fig. 5).
NMDS bubble plot comparing the recruitment structure (average of number of recruits (cyprids plus juveniles RC < 0.6 mm) per barnacle per site in each date) at each of four regions (3 sites in Brittany, 6 sites in Asturias, 6 sites in Galicia, 5 sites in SW Portugal) based on Bray–Curtis similarities of square-root transformed data. Vectors indicate direction and magnitude of Pearson correlation coefficient between the variable (number of recruits per barnacle in each date, averaged by site) and the MDS axes (only variables with |r|> 0.7 were included). The size of the bubbles indicates the average size (RC, mm) of the barnacles sampled in each site across the study period
Reproduction, recruitment and environmental variation
Different regional patterns regarding the lag between the beginning of the main reproduction season and the beginning of the main recruitment season in 2018 (the only complete year) were found: 1 month in Brittany (here we considered July as the start of the main recruitment season, the first month in 2018 with ≥ 1 recruit per adult); 2 months in Asturias and Galicia; 4 months in SW Portugal (Fig. 7).
Phenology and intensity of reproduction (Rep) and recruitment (Rec) for 2018 (the only complete year) in Brittany, Asturias, Galicia and SW Portugal. Light grey stands for the non-breeding and no-recruitment seasons, and black for the main reproductive and main recruitment seasons. Data for blank cells (January and December in Brittany) are missing
Regarding the variation of environmental variables during the study period (Fig. 8 and Table S8 in SSMM), different patterns can be distinguished among regions in relation to the average monthly temperatures. While in Asturias, Galicia and SW Portugal considerable differences between SST and air temperature were recorded throughout the year, in Brittany SST and air temperature remained fairly similar (Fig. 8b). The most striking patterns relatively to air temperature are summer being warmer in SW Portugal (average of 21 ºC and of 18–18.9 ºC in other regions) and winter being colder in Brittany, Asturias and Galicia (average values from 9 to 10.5 ºC) than SW Portugal (13 ºC) (Fig. 8b and Table S8 in SSMM).
In relation to SST, Brittany registered the lowest average of SST in winter (10 ºC (Brittany), ≥ 12.6 (other regions)), in spring (13.8 ºC (Brittany), ≥ 14.8 ºC (other regions)) and in autumn (13.2 ºC (Brittany), ≥ 14.5 ºC (other regions)) (see Table S8 in SSMM for a description of the seasonal means per region). However, in summer the mean SST in Brittany was considerably higher (17.2 ºC), similar to SW Portugal (17.5 ºC) and warmer than in Galicia (16.4 ºC). The lower average SST values in Brittany from autumn to spring seem to be related to a longer non-breeding season in this region, while a warmer summer seems to be linked to the breeding season (Fig. 8a, b).
Another pattern that can be described is a gradual loss of seasonality of SST from the northern locations (Brittany) towards Asturias, Galicia and SW Portugal (Fig. 8b). Mean annual SST was higher in SW Portugal (16.3 ºC) and Asturias (15.4 ºC) and lower in Galicia (14.5 ºC) and Brittany (13.4 ºC). This regional pattern of SST was observed in autumn (SW Portugal, 17.3 ºC, Asturias, 15.6 ºC, Galicia, 14.5 ºC and Brittany, 13.2 ºC)), but not in winter and spring when a north to south increase was observed, and in summer where a different SST pattern was observed: Asturias (19.1 ºC), Portugal (17.6 ºC), Brittany (17.2 ºC), and Galicia (16.4 ºC) (Table S8 in SSMM).
Concerning chlorophyll-a, the lowest seasonal average values were observed in Asturias (≤ 1.5 mg m−3 in all seasons) (Table S8 in SSMM). In Brittany and Portugal, a pronounced seasonality was observed, with the average chlorophyll-a being highest in spring and summer (> 3 mg m−3) and lowest in autumn and winter (< 1.8 mg m−3). In Galicia, the average chlorophyll-a was ≥ 2 mg m−3 in all seasons, being higher in autumn and winter (≥ 3 mg m−3) and being higher than in other regions in autumn and winter (Fig. 8c and Table S8 in SSMM).
The mean upwelling index remained positive both annually and across seasons in SW Portugal (Fig. 8d). However, it should be noted that in SW Portugal, the seasonal average values were considerably lower and indicative of lower intensity of upwelling/downwelling in autumn (64 m3 s−1 km−1 versus 740 m3 s−1 km−1 in summer, Table S8 in SSMM). In Asturias and Galicia, the mean upwelling index values experienced greater fluctuations, ranging from primarily downwelling episodes in winter and autumn (negative values) to upwelling events in spring and summer (positive values) (Fig. 8d and Table S8 in SSMM).
Discussion
Through a large-scale standardized comparison of the reproduction and recruitment of P. pollicipes across Europe, we have found regional differences in the phenology and intensity of both biological processes. The most striking patterns were a lower reproductive effort in northern (Brittany) than in Iberian populations (Asturias, Galicia and SW Portugal), and a contradictory pattern in Galicia characterised by the absence of a non-breeding period and by a shorter main recruitment season than observed in other Iberian regions.
In Brittany, a significant lower reproductive effort was observed in comparison with the Iberian regions. This was mainly characterised by a longer non-breeding season (7–9 months) and a shorter main reproductive season (i.e., when most of the barnacles had eggs: June, July and August); which might have been caused by favourable thermal conditions only in summer. The average SST in Brittany was lower than in the Iberian Peninsula in autumn, winter and spring, while it was similar to SW Portugal and higher than Galicia in summer.
As sampled sites in Brittany are located close to the northern limit of the distribution of P. pollicipes (see Fig. 1a, b), it is possible to consider that this regional reproductive pattern supports the prediction of a lower population performance in peripheral populations according to the “centre–periphery hypothesis” (Pironon et al. 2016). This paradigm includes the idea that environmental conditions are optimal near the centre of the species range and suboptimal at the periphery (Brown 1984; Pironon et al. 2016). Temperature is a major environmental factor influencing physiology and ecology of marine species. As P. pollicipes is a warm-temperate species we might expect physiological constraints of the reproductive process imposed by low temperatures, as was previously reported for other crustaceans (Van Den Brink et al. 2012; Green et al. 2014). Short breeding periods restricted to summer were also observed in northern population of other warm-temperate intertidal species in Europe (e.g., Patella ulyssiponensis, Lewis 1986). In addition, the longer non-breeding season observed in Brittany might be caused by geographical differences in the male gonad development. In fact, previous studies on the gonadal development of P. pollicipes in Galicia and SW Portugal have indicated that the reproductive season of this species solely depends upon the development of the female gonad, as spermatozoa were stored in the seminal vesicles during all year (Molares et al. 1994a; Cruz and Hawkins 1998). Contrarily, Barnes (1992) rarely observed spermatozoa from October to March in P. pollicipes collected at Biarritz (France). On the other hand, the high primary productivity that was observed in Brittany in spring (mean chlorophyll-a values of 5.5 mg m−3) may have triggered gonad maturation in spring and subsequent fertilization in summer.
The low reproductive effort in Brittany might also explain the low recruitment rates found in this region due to a potential decrease in larval supply, intensified by: the absence of P. pollicipes north of Brittany and on most of the French coast south of Brittany as a consequence of lack of suitable habitat; and the longer distance larvae needs to travel from other spawning populations (e.g., Iberian Peninsula) (see Fig. 1a). Low recruitment rates might also be driven by local hydrodynamics that affect larval transport in south Brittany (see Ayata et al 2010). Previous results from a 3D biophysical model for south Brittany showed that larval dispersal is mainly oriented southwards to offshore waters on short distances (i.e., few tens of km for a 4-week planktonic larval duration, PLD) from June to August (Ayata et al 2010). This offshore transport might also explain the low recruitment intensity observed during summer in Brittany. Our results are similar to previous studies on the reproduction of this species during spring and summer in Brittany (De Kergariou 1971 in Girard 1982; Joncourt 2005).
Lower reproductive effort and the low values of recruitment intensity in Brittany might have important implications for fisheries management. Despite our observations of a high cover of P. pollicipes in Brittany (unpublished observations), probably related to a low fishing intensity in this region (Aguión et al. 2022), under a scenario of more fishing effort, the recovery of populations in Brittany may be more difficult. However, this hypothetical negative trend could be reversed in the context of climate change, if reproductive and recruitment effort increases as a consequence of global warming or becomes more similar to the current patterns we have observed on the Iberian Peninsula. In fact, several benthic invertebrates species are already expanding their poleward range limits as a response to warming (Hiddink et al. 2015; Poloczanska et al. 2016).
The Iberian populations of P. pollicipes had a main reproductive season that lasted for 5 months (from May/June to September/October in Asturias and Galicia depending on the year, and from April to August in SW Portugal). These timings match previous observations of the species reproduction in the Iberian Peninsula (De la Hoz and García 1993; Cardoso and Yule 1995; Cruz 2000; Pavón 2003; Macho 2006) although a 1-month earlier start was recorded in SW Portugal. In fact, in studies conducted in the early 1990’s, the majority of the population in SW Portugal were only observed to have ≥ 50% of barnacles with eggs from May onwards (Cardoso and Yule 1995; Cruz and Hawkins 1998; Cruz 2000).
In SW Portugal, the end of the main reproduction season was earlier (August) than in Asturias and Galicia (September or October), which means that fertilization might drop in the end of summer in SW Portugal. Considering the environmental variables that were measured in the Iberian Peninsula, the most obvious regional difference during summer is in air temperature that is higher in SW Portugal (average of 21 ºC) than in Asturias and Galicia (average of 18.2 ºC and 18.9 ºC, respectively). The apparently positive relationship between air temperature and the percentage of barnacles with eggs in SW Portugal may result from an inhibition of the female gonad development or of the fecundation process at higher atmospheric temperatures such as those recorded in summer in SW Portugal. One hypothesis is that the fecundation rate would decrease in summer in SW Portugal, resulting in a pronounced decrease in the percentage of barnacles with eggs in late summer comparing to Asturias and Galicia. Air temperature has also been considered to have a positive effect in the reproduction of P. pollicipes in SW Portugal (Cardoso and Yule 1995) and of Chthamalus montagui (Barnes 1992).
The higher annual reproduction intensity found in Galicia, that includes the absence of a non-breeding season in this region, might be explained by more favorable food conditions in Galicia, as average chlorophyll-a values were equal to or greater than 2 mg m−3 in all seasons, being higher than those observed in the other regions in autumn and winter. This higher primary productivity in autumn and winter might be a proxy of more food and related to maintaining some investment in reproduction during the whole year.
The annual number of broods estimated in the present study varied depending on the assumption of a fixed embryo development time (25 days) (as in previous studies, e.g., Cruz and Araújo 1999; Macho 2006) or of an embryo development time dependent on water temperature (new methodology, see Supplementary material related to number of broods estimation). Using the traditional methodology, the number of broods varied from 3 in Brittany to 5 in Asturias and SW Portugal, and to 6 in Galicia. These estimates are higher than the ones estimated in the past using a similar methodology (4 in SW Portugal, Cruz and Araújo 1999; 5 in Galicia, Macho 2006). If we consider a relationship between embryo development and water temperature, these estimates decreased and ranged from 2 in Brittany to 4 in Iberian regions. We consider the latter estimates to be more realistic, taking into account the influence of water temperature on embryonic development time of barnacles (Patel and Crisp 1960). Again, we found a decrease in the reproductive effort in northern populations in comparison with Iberian populations.
Recruitment patterns of P. pollicipes in the Iberian Peninsula were considered significantly different between Galicia and Asturias and SW Portugal, mostly due to the lower values of recruitment observed during autumn in Galicia and of an overall shorter main recruitment season in this region. The main recruitment season lasted between 4 and 6 months in Asturias and SW Portugal (July to October/December in Asturias, and July/August to November/January in SW Portugal), while in Galicia just 2 months of the study period surpass the threshold of > 3 recruits used to define it (July 2017 and August 2018). Previous studies on P. pollicipes also defined summer and autumn as the main recruitment season of the species, although important inter-annual variability has been reported (Pavón 2003; Macho 2006; Cruz et al. 2010; Fernandes et al. 2021). The average of recruits per adult in SW Portugal matches previous observations of this species (Cruz et al. 2010; Fernandes et al. 2021, although the recruitment index was cyprids plus juveniles < 1 mm RC instead of < 0.6 mm). In Asturias, inter-annual variation in recruitment was observed in 2000 and 2001, with recruitment values being lower in 2000 and similar in 2001 to those observed in the present study (Pavón 2003).
The patterns of reproduction and recruitment found in Galicia are contradictory, since considering the three Iberian regions studied, Galicia is the region that theoretically seems to provide more larvae (higher reproductive effort) and receive less (lower recruitment values, particularly in autumn, if less recruitment is indicative of a less larval supply). In Galicia, the non-extension of the main recruitment season into autumn (as is the case in Asturias and SW Portugal) may be due to the lower mean SST found during autumn in this region (14.5 ºC in Galicia; 15.6 ºC in Asturias; 17.3 ºC in SW Portugal). The lower SST recorded in Galicia does not seem to be related to a higher upwelling intensity in this region, because the upwelling mean in autumn was higher in SW Portugal (64) than in Galicia (− 458) and Asturias (− 634).
The positive relationship between recruitment and SST suggested in our study (Asturias and Portugal vs. Galicia) has been previously observed in a 10-year study monitoring the recruitment of P. pollicipes in Cape of Sines (SW Portugal) (Fernandes et al. 2021). In this study, a clear association between recruitment and both relaxation of upwelling and seawater warming was detected. In fact, as revised in Fernandes et al. (2021), SST might have an indirect effect on recruitment by being a proxy of upwelling relaxation and promoting onshore or along shore larval transport, or by being a proxy of water stratification and enhancing onshore propagation of internal motions. As there is apparently no relationship between the lower temperature in autumn SST in Galicia and a higher upwelling intensity, we may speculate that the lower autumn temperature in Galicia may be an indication that other processes favourable to recruitment and on which we have no data (e.g., internal motions) are not occurring. In addition, a higher SST can increase pelagic larval survival of acorn barnacles (Scrosati and Ellrich 2016) and warming might have an effect on reducing the planktonic larval duration of P. pollicipes (Franco et al. 2017). Although all sites are similar with respect to wave exposure (very exposed sites), the topographic complexity on a regional scale is different between Galicia and the other regions due to the presence of “rias” in Galicia. However, there are no studies so far on the influence of the “rias” in the recruitment of intertidal invertebrates with larval phases in Galicia. The regional variation detected between Galicia and Asturias and SW Portugal does not seem to be due to different fishing efforts among regions. To our knowledge, none of the fisheries seem overfished (see Aguión et al 2022), although there are no stock assessment of P. pollicipes on these regions. Unravelling the processes (pre and post-settlement) that are driving the lower annual recruitment intensity in Galicia requires further investigation and it is important in a context of climate change with practical implications in the ecology and management of stalked barnacles. The projected weakening of upwelling in the Iberian Peninsula (Pires et al. 2016; Sousa et al. 2020) and the increasing warmer waters, are likely to promote a longer and more intense recruitment season for P. pollicipes in SW Portugal (Fernandes et al. 2021).
When we compared the start of the main reproduction season with the start of the main recruitment season, we observed that there was an increasing interval from north to south; 1 month in Brittany, 2 months in Asturias and Galicia and 4 months in Portugal. This 4-month interval in SW Portugal was already observed in this region (Cruz 2000), and might be caused by offshore larval transport mediated by upwelling during spring and beginning of summer. Indeed, according to a biophysical model of larval dispersal of P. pollicipes in the Iberian Peninsula recently developed (Nolasco et al. submitted), larvae from the central (advected southward) and southwest (advected westward) coast of Portugal are accumulated off SW Portugal. According to this model, upwelling also carries the larvae far offshore in Galicia, and in both Galicia and SW Portugal, the offshore larval pool is then advected onshore during upwelling relaxation. As the main reproduction season begins earlier in SW Portugal (April) (mature eggs have been observed in SW Portugal since March), than in Galicia (June) (mature eggs since May), it might be that this upwelling “barrier” will have a more lasting effect in SW Portugal than in Galicia. The upwelling index calculated for Cape of Sines (SW Portugal) during 10 years (2007–2016) generally increased during spring until July or August and decreased during the rest of summer and autumn (Fernandes et al. 2021). The main recruitment season in 2018 in both Galicia and SW Portugal began in August and the upwelling index decreased after August. In Galicia P. pollicipes mature eggs have been found as early as May (this work and Macho 2006), but last nauplii stages were not found in the water in coastal stations until August (Macho et al. 2010), in line with this upwelling “barrier” effect during spring and most of summer, coinciding with the upwelling season in Galicia (Álvarez et al. 2008). In addition, it is highly unlikely that the increasing interval from north to south is associated with a longer PLD further south. In laboratory conditions, the median development time of P. pollicipes cypris at 15 ºC and at 20 ºC was 18 and 15.5 days, respectively (Franco et al. 2017), although cyprids might live for longer periods with mortality of about 50% after 20 days (Franco et al. 2016). As there is evidence of a negative effect of temperature on PLD (e.g., O’Connor et al. 2007; Franco et al. 2017), considering the temperature gradient from Britany to SW Portugal (Brittany, mean value of 13.8 ºC in spring and 17.2 ºC in summer; SW Portugal, mean value of 15.8 ºC in spring and 17.6 ºC in summer), it seems not plausible that PLD is longer in SW Portugal than in Brittany.
In conclusion, using a standardised methodology we compared the reproductive and recruitment patterns of P. pollicipes in Europe. We highlight the lower reproductive and recruitment effort that was detected near the northern limit of this species (Brittany), as well as the potential negative (in the case of an increase in fishing of this resource) and positive (relatively to climate change) impacts that might occur on this species in this region. Regarding the variation of these processes in the Iberian regions, there seem to be more similarities between Asturias and SW Portugal than with Galicia. The Galician region showed a contradictory pattern of higher reproductive effort and a lower intensity of recruitment that should be investigated in the future. In the future, it would also be very interesting to model the response of these processes to environmental variability, testing some of the relationships that were apparent in the descriptive analysis we made, as well as analysing the variability of these processes at a smaller spatial scale (among sites).
To increase our knowledge on the ecosystem resilience of marine populations, a renewed paradigm calls for the need to scale-up ecological studies (Hughes et al. 2005). The management of resources like the stalked barnacle is usually fragmented at smaller scales (Aguión et al. 2022), and a better understanding of population dynamics and their responses to large-scale environmental changes is needed to support nested governance that accounts for internal (i.e., market dynamics, local heterogeneity, etc.) and external (i.e., climate change) drivers (Hughes et al. 2005). For this, further studies conducted at large spatial scales and that use standardised protocols are needed for adequate comparisons of biological processes across the distribution area of species.
Availability of data and material
Data are available from the authors on request.
Code availability
Not Applicable.
References
Achituv Y, Barnes H (1978) Studies in the biochemistry of cirripede eggs.VI.Changes in the general biochemical composition during development of Tetraclita squamosa rufotincta Pilsbry, Balanus perforatus Brug., and Pollicipes cornucopia Darwin. J Exp Mar Bio Ecol 32:171–176
Aguión A, Ojea E, García-Flórez L, Cruz T, Garmendia JM, Davoult D, Queiroga H, Rivera A, Acuña-Fernández JL, Macho G (2022) Establishing a governance threshold in small-scale fisheries to achieve sustainability. Ambio 51(3):652–665
Álvarez I, Gomez-Gesteira M, deCastro M, Dias JM (2008) Spatio-temporal 667 evolution of upwelling regime along the western coast of the Iberian Península. J Geophys Res 113:C07020
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Ayata SD, Lazure P, Thiébaut E (2010) How does the connectivity between populations mediate range limits of marine invertebrates? A case study of larval dispersal between the Bay of Biscay and the English Channel (North-East Atlantic). Progr Oceanogr 87:18–36
Barnes M (1992) The reproductive periods and condition of the penis in several species of common cirripedes. Oceanogr Mar Biol Annu Rev 30:483–525
Barnes M (1996) Pedunculate cirripedes of the genus Pollicipes. Oceanogr Mar Biol Annu Rev 34:303–394
Broitman BR, Mieszkowska N, Helmuth B, Blanchette CA (2008) Climate and recruitment of rocky shore intertidal invertebrates in the Eastern North Atlantic. Ecology 89:81–90
Brown JH (1984) On the relationship between abundance and distribution of species. Am Nat 124:255–279
Brown JH, Stevens GC, Kaufman DM (1996) The geographic range: size, shape, boundaries, and internal structure. Annu Rev Ecol Syst 27:597–623
Cardoso AC, Yule AB (1995) Aspects of the reproductive biology of Pollicipes pollicipes (Cirripedia; Lepadomorpha) from the southwest coast of Portugal. Neth J Aquat Ecol 29:391–396
Clarke KR, Gorley RN (2015) Getting started with PRIMER v7. PRIMER-E. Plymouth Marine Laboratory, Plymouth
Connell JH (1985) The consequences of variation in initial settlement vs. post-settlement mortality in rocky intertidal communities. J Exp Mar Biol Ecol 93:11–45
Connolly SR, Menge BA, Roughgarden J (2001) A latitudinal gradient in recruitment of intertidal invertebrates in the Northeast Pacific Ocean. Ecology 82:1799–1813
Crisp DJ, Southward AJ, Southward EC (1981) On the distribution of the intertidal barnacles Chthamalus stellatus, Chthamalus montagui and Euraphia depressa. J Mar Biol Assoc UK 61:359–380
Cruz T (2000) Biologia e ecologia do percebe, Pollicipes pollicipes (Gmelin, 1790), no litoral sudoeste português. PhD thesis, Universidade de Évora, Portugal
Cruz T, Araújo J (1999) Reproductive patterns of Pollicipes pollicipes (CIRRIPEDIA: SCALPELLOMORPHA) on the Southwest coast of Portugal. J Crustac Biol 19:260–267
Cruz T, Hawkins SJ (1998) Reproductive cycle of Pollicipes pollicipes at Cabo de Sines, south-west coast of Portugal. J Mar Biol Assoc UK 78:483–496
Cruz T, Castro JJ, Hawkins SJ (2010) Recruitment, growth and population size structure of Pollicipes pollicipes in SW Portugal. J Exp Mar Biol Ecol 392:200–209
Cruz T, Jacinto D, Sousa A, Penteado N, Pereira D, Fernandes JN, Silva T, Castro JJ (2015) The state of the fishery, conservation and management of the stalked barnacle Pollicipes pollicipes in Portugal. Mar Environ Res 112:73–80
Cruz T, Jacinto D, Fernandes JN, Seabra MI, Van Syoc RJ, Power AM, Macho G, Sousa A, Castro JJ, Hawkins SJ (2022) Pedunculate cirripedes of the genus Pollicipes: 25 years after Margaret Barnes review. Oceanogr Mar Biol: an annual review (accepted)
De la Hoz JJ, Garcia L (1993) Datos para el estudio de la distribución y reproducción del percebe, Pollicipes cornucopiae (Leach), en Asturias. Publ Espec Inst Oceanogr 11
De Kergariou G (1971) Le pouce-pied (Pollicipes cornucopiae Leach) sur les côtes de Belle-Île. Etude préliminaire – ISTPM
Fernandes JN, Jacinto D, Penteado N, Sousa A, Mateus D, Seabra MI, Silva T, Castellanos P, Castro JJ, Cruz T (2021) Ten years of monitoring recruitment of the edible stalked barnacle Pollicipes pollicipes: linking to oceanographic variability. Limnol Oceanogr 66:2309–2318
Franco SC, Aldred N, Cruz T, Clare AS (2016) Modulation of gregarious settlement of the stalked barnacle, Pollicipes pollicipes: a laboratory study. Sci Mar 80(2): 217–228
Franco SC, Aldred N, Cruz T, Clare AS (2017) Effects of culture conditions on larval growth and survival of stalked barnacles (Pollicipes pollicipes). Aquac Res 48:2920–2933
Fredston A, Pinsky M, Selden RL, Szuwalski C, Thorson JT, Gaines SD, Halpern BS (2021) Range edges of North American marine species are tracking temperature over decades. Glob Change Biol 27:3145–3156
Gaines S, Roughgarden J (1985) Larval settlement rate: A leading determinant of structure in an ecological community of the marine intertidal zone. Proc Natl Acad Sci USA 82:3707–3711
Gaston KJ (2009) Geographic range limits: achieving synthesis. Proc R Soc B 276:1395–1406
Gaylord B, Gaines SD (2000) Temperature or transport? Range limits in marine species mediated solely by flow. Am Nat 155:769–789
Gilman SE (2006) The northern geographic range limit of the intertidal limpet Collisella scabra: a test of performance, recruitment, and temperature hypothesis. Ecography 29:709–720
Girard S (1982) Étude du stock de pouces-pieds de Belle-Île et de son exploitation. Mémoire de fin d'études ENSAR
Green BS, Gardner C, Hochmuth JD, Linnane A (2014) Environmental effects on fished lobster and crabs. Rev Fish Biol Fisheries 24:613–638
Hawkins SJ, Pack KE, Firth LB, Mieszkowska N, Evans AJ, Martins GM, Åberg P, Adams LC, Arenas F, Boaventura DM, Bohn K, Borges CDG, Castro JJ, Coleman RA, Crowe TP, Cruz T, Davies MS, Epstein G, Faria J, Ferreira JG, Frost NJ, Griffin JN, Hanley ME, Herbert RJH, Hyder K, Johnson MP, Lima FP, Masterson-Algar P, Moore PJ, Moschella PS, Notman GM, Pannacciulli FG, Ribeiro PA, Santos AM, Silva ACF, Skov MW, Sugden H, Vale M, Wangkulangkul K, Wort EJG, Thompson RC, Hartnoll RG, Burrow MT, Jenkins SR (2019) The intertidal zone of the North-East Atlantic Region: pattern and process in interactions in the marine benthos, global patterns and processes. In: Hawkins SJ, Bohn K, Firth LB, Williams GA (eds) Systematics association special, vol 87. Cambridge University Press
Herrera M, Wethey DS, Vázquez E, Macho G (2019) Climate change implications for reproductive success: temperature effect on penis development in the barnacle Semibalanus balanoides. Mar Ecol Prog Ser 610:109–123
Hiddink JG, Burrows MT, Molinos JG (2015) Temperature tracking by North Sea benthic invertebrates in response to climate change. Glob Change Biol 21:117–129
Hilgard GH (1960) A study of reproduction in the intertidal barnacle, Mitella polymerus, in Monterey bay, California. Biol Bull 119:168–188
Hughes TP, Bellwood DR, Folke C, Steneck RS, Wilson J (2005) New paradigms for supporting the resilience of marine ecosystems. Trends Ecol Evol 20:380–386
Joncourt Y (2005) Étude socio-biologique appliquée à la gestion d’une pêcherie de loisir: cas des pouces-pieds (Mitella pollicipes de Saint-Guénolé. Master Mention Biologie Fondamentale et Appliquée. Université de Caen
Kugele M, Yule AB (1996) The larval morphology of Pollicipes pollicipes (Gmelin, 1790) (Cirripedia: Lepadomorpha) with notes on cypris settlement. Sci Mar 60: 469–480
Lewis JR (1986) Latitudinal trends in reproduction, recruitment and population characteristics of some rocky littoral molluscs and cirripedes. Hydrobiologia 142:1–13
Macho G (2006) Ecología reproductiva y larvaria del percebe y otros cirrípedos en Galicia. PhD thesis, Universidade de Vigo, Spain
Macho G, Vázquez E, Giráldez R, Molares J (2010) Spatial and temporal distribution of barnacle larvae in the partially mixed estuary of the Ría de Arousa (Spain). J Exp Mar Biol Ecol 392:129–139
Mateus D (2017) Variabilidade espacial e temporal do recrutamento de Pollicipes pollicipes na região de Sines. MSc thesis, Universidade de Aveiro, Portugal
Mieszkowska N, Kendall MA, Hawkins SJ, Leaper R, Williamson P, Hardman-Mountford NJ, Southward AJ (2006) Changes in the range of some common rocky shore species in Britain – a response to climate change? Hydrobiologia 555:241–251
Molares J, Freire J (2003) Development and perspectives for community-based management of the gooseneck barnacle (Pollicipes pollicipes) fisheries in Galicia (NW Spain). Fish Res 65:485–492
Molares J, Quintana R, Rodríguez S (1994a) Gametogenesis of Pollicipes cornucopia (Cirripedia: Scalpellomorpha) in North-West Spain. Mar Biol 120:553–560
Molares J, Tilves F, Pascual C (1994b) Larval development of the pedunculate barnacle Pollicipes cornucopia (Cirripedia: Scalpellomorpha) reared in the laboratory. Mar Biol 120:261–264
Molares J (1993) Estudio del ciclo biológico del percebe (Pollicipes cornucopia LEACH) de las costas de Galicia. PhD, Universidade de Santiago de Compostela, Spain
Moore PJ, Thompson RC, Hawkins SJ (2011) Phenological changes in intertidal con-specific gastropods in response to climate warning. Glob Change Biol 17:709–719
O’Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM (2007) Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci USA 104(4):1266–1271
O’Riordan RM, Arenas F, Arrontes J, Castro JJ, Cruz T, Delany J, Martínez B, Fernandez C, Hawkins SJ, McGrath D, Myers AA, Oliveros J, Pannacciulli FG, Power AM, Relini G, Rico JM, Silva T (2004) Spatial variation in the recruitment of the intertidal barnacles Chthamalus montagui Southward and Chthamalus stellatus (Poli) (Crustacea: Cirripedia) over an European scale. J Exp Mar Biol Ecol 304:243–264
Patel BS, Crisp DD (1960) Rates of development of embryos of several species of barnacle. Physiol Zool 33:104–119
Pavón C (2003) Biología y variables poblacionales del percebe, Pollicipes pollicipes (Gmelin, 1970) en Asturias. PhD thesis, Universidad de Oviedo, Spain
Philippart CJM, Amaral A, Asmus R, van Bleijswijk J, Bremmer J, Buchholz F, Cabanellas-Reboredo M, Catarino D, Cattrijsee A, Charles F, Comtet T, Cunha A, Deudero S, Duchene FS, Gentil F, Gittenberger A, Guizien K, Gonçalves JM, Guarnieri G, Hendriks I, Hussel B, Pinheiro Vieira R, Reijnen BT, Sampaio I, Serrao E, Sousa Pinto I, Thiebaut E, Viard F, Zuur AF (2012) Spatial synchronies in the seasonal occurrence of larvae of oysters (Crassotea gigas) and mussels (Mytilus edulis/galloprovincialis) in European coastal waters. Estuar Coast Shelf Sci 108:52–63
Pineda J (2000) Linking larval settlement to larval transport: assumptions, potentials and pitfalls. Oceanogr Eastern Pac I:84–105
Pires AC, Nolasco R, Rocha A, Ramos AM, Dubert J (2016) Climate change in the Iberian Upwelling System: a numerical study using GCM downscaling. Clim Dyn 47:451–464
Pironon S, Papuga G, Villellas J, Angert AL, García MB, Thompson JD (2016) Geographic variation in genetic and demographic performance: new insights from an old biogeographical paradigm. Biol Rev 92:1877–1909
Poloczanska ES, Burrows MT, Brown CJ, Molinos JG, Halpern BS, Hoegh-Guldberg O, Kappel CV, Moore PJ, Richardson AJ, Schoeman DS, Sydeman WJ (2016) Responses of marine organisms to climate change across oceans. Front Mar Sci 3:62
Rivadeneria MM, Hernáez P, Baeza JA, Boltaña S, Cifuentes M, Correa C, Cuevas A, del Valle E, Hinojosa I, Ulrich N, Valdivia N, Vásquez N, Zander A, Thiel M (2010) Testing the abundant-centre hypothesis using intertidal porcelain crabs along the Chilean Coast: linking abundance and life-history variation. J Biogeogr 37:486–498
Sagarin RD, Gaines SD (2002) Geographical abundance distributions of coastal invertebrates: using one-dimensional ranges to test biogeographic hypothesis. J Biogeogr 29:985–997
Scrosati RA, Ellrich JA (2016) A 12-year record of intertidal barnacle recruitment in Atlantic Canada (2005–2016): relationships with sea surface temperature and phytoplankton abundance. Peer J 4:e2623
Sousa MG, Ribeiro A, Des M, Gomez-Gesteira M, de Castro M, Dias JM (2020) NW Iberian Peninsula coastal upwelling future weakening: Competition between wind intensification and surface heating. Sci Total Environ 703:134808
Southward AJ (2008) Barnacles: keys and notes for the identification of British species. In: Crothers JH, Hayward PJ (eds) Synopses of the British Fauna (New Series). Linnean Society of London, The Estuarine and Coastal Sciences Association and Field Studies Council, Shrewsbury, p 140
Van den Brink AM, McLay CL, Hosie AM, Dunnington MJ (2012) The effect of temperature on brood duration in three Halicarcinus species (Crustacea: Brachyura: Hymenosomatidae). J Mar Biol Assoc UK 92:515–520
Acknowledgements
We thank all the fishers from Brittany, Asturias, Galicia and SW Portugal, and biologists and surveillance officers (from cofradías and regional fisheries administration) from Asturias and Galicia that worked with us. We would like to thank two anonymous reviewers and the editor for their careful reading of our manuscript and their insightful comments. This research was funded through the 2015-2016 BiodivERsA COFUND Theme 2 call for research proposals, with the national funders Agencia Estatal de Investigación, Spain (grants PCIN-2016-120 to JLA, University of Oviedo and PCIN-2016-063 to EV, University of Vigo), Fundação para a Ciência e Tecnologia, Portugal (grants BIODIVERSA/0005/2015 to TC, University of Evora and BIODIVERSA/0006/2015 to JD, University of Aveiro) and the Agence Nationale de la Recherche, France (grants ANR-16-EBI3-0006-01 to DD, Sorbonne University and ANR-16-EBI3-0006-02 to AN, ENSTA Bretagne). This is a contribution of the Asturias Marine Observatory. MARE—Marine and Environmental Sciences Centre had the support of FCT through the strategic project UIDB/04292/2020—MARE. Thanks are also due to FCT/MCTES for the financial support to CESAM (UIDP/50017/2020+UIDB/50017/2020), through national funds. AA is supported by a FPU fellowship (Ref. FPU2016-04258, Spanish Ministry of Science, Innovation and Universities). KG is supported by a fellowship from the Severo Ochoa PhD program (Ref. PA-18-PF-BP17-184, Principado de Asturias). GM is supported by post-doctoral contracts from projects PERCEBES (PCIN-2016-063) and MARISCO (CTM2014-51935-R, Spanish Ministerio de Economía y Competitividad). Funding for open access charge: Universidade de Vigo/CISUG.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded through the 2015–2016 BiodivERsA COFUND Theme 2 call for research proposals, with the national funders Agencia Estatal de Investigación, Spain (grants PCIN-2016–120 to JLA, University of Oviedo and PCIN-2016–063 to EV, University of Vigo), Fundação para a Ciência e Tecnologia, Portugal (grants BIODIVERSA/0005/2015 to TC, University of Evora and BIODIVERSA/0006/2015 to JD, University of Aveiro) and the Agence Nationale de la Recherche, France (grants ANR-16-EBI3-0006–01 to DD, Sorbonne University and ANR-16-EBI3-0006–02 to AN, ENSTA Bretagne).
Author information
Authors and Affiliations
Contributions
AA, TC, JLA, ET and GM conceptualized the research. TC, JLA, DD, EV and GM acquired funding. TC, JLA, DD, JD, ET, EV and GM were project administrators. AA, TC, JLA, CB, JC, JF, KG, DJ, DM, ST, ET and GM designed the methodology. Data curation, formal analysis and the original draft were prepared by AA and TC. Visualization was prepared by AA, TC and GM. All authors participated in the investigation process and contributed to review and editing. TC and GM supervised the study.
Corresponding author
Ethics declarations
Conflict of interest/Competing interests
Authors do not have any conflict of interest.
Ethics approval
Not Applicable.
Consent to participate
All authors agree on their participation on this paper.
Consent for publication
All authors agree to be co-authors in the order submitted.
Additional information
Responsible Editor: S. Connell.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alba Aguión and Teresa Cruz are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aguión, A., Cruz, T., Acuña, J.L. et al. A large-scale comparison of reproduction and recruitment of the stalked barnacle Pollicipes pollicipes across Europe. Mar Biol 169, 63 (2022). https://doi.org/10.1007/s00227-022-04050-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04050-x