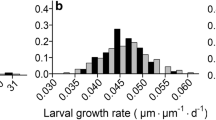
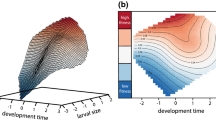
Abstract
Growth rate affects body size, and larger body sizes are often associated with the capacity to produce more surviving offspring. However, the assumption that growth rate should positively relate to fitness is rarely tested, especially in colonial marine invertebrates where size and age can be decoupled. We measured growth, survival, and reproduction through repeated census of 97 colonies from two populations of a marine bryozoan in the field from settlement to the end of their reproductive season in the northern Gulf of Mexico. Despite large population differences in fitness when grown in a common garden setting, selection within populations on variation in relative growth rate prior to reproduction was similar. In both populations, colonies that grew faster early after settlement, hence were larger, did not consistently have higher fitness than colonies that grew slower after settlement. Instead, early juvenile growth was uncorrelated to later juvenile growth, and colonies that grew faster just prior to the onset of reproduction had higher fitness than colonies that grew slower during this time. Growth rates then declined with the onset of reproduction. Our results show that, rather than being a simple consequence of selection on body size, growth rate can directly affect variation in fitness in ways that are not directly attributable to juvenile size. Colony size and growth rate in modular animals are not always reliable surrogates for direct estimates of survival and reproduction, without identifying when and how growth affects fitness.


Similar content being viewed by others
Data accessibility
Data are available on the Dryad Digital Repository at https://doi.org/10.5061/dryad.j9kd51cb2
Code availability
R Code to reproduce the statistical results and plots is available on the Dryad Digital Repository at https://doi.org/10.5061/dryad.j9kd51cb2
References
Allen R, Buckley Y, Marshall D (2008) Offspring size plasticity in response to intraspecific competition: an adaptive maternal effect across life-history stages. Am Nat 171:225–237
Álvarez-Noriega M, Baird AH, Dornelas M, Madin JS, Cumbo VR, Connolly SR (2016) Fecundity and the demographic strategies of coral morphologies. Ecology 97:3485–3493
Bishop JDD, Pemberton AJ (2006) The third way: Spermcast mating in sessile marine invertebrates. Integr Comp Biol 46:398–406
Blanckenhorn WU (2000) The evolution of body size: what keeps organisms small? Q Rev Biol 75:385–407
Burgess SC, Marshall DJ (2011a) Are numbers enough? Colonizer phenotype and abundance interact to affect population dynamics. J Anim Ecol 80:681–687
Burgess SC, Marshall DJ (2011b) Field estimates of planktonic larval duration in a marine invertebrate. Mar Ecol Prog Ser 440:151–161
Burgess SC, Bode M, Marshall DJ (2013) Costs of dispersal alter optimal offspring size in patchy habitats: combining theory and data for a marine invertebrate. Funct Ecol 27:757–765
Burgess SC, Ryan WH, Blackstone NW, Edmunds PJ, Hoogenboom MO, Levitan DR, Wulff JL (2017) Metabolic scaling in modular animals. Invertebr Biol 136:456–472
Chadwick-Furman NE, Weissman IL (1995) Life history plasticity in chimaeras of the colonial ascidian Botryllus schlosseri. Proc R Soc B 262:157–162
Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Côté IM, Bellwood D (2012) Evaluating life-history strategies of reef corals from species traits. Ecol Lett 15:1378–1386
Denley D, Metaxas A (2017) Effects of intrinsic and extrinsic factors on reproduction of an ecologically significant invasive bryozoan: implications for invasion success. Mar Biol 164:145
Dmitriew CM (2011) The evolution of growth trajectories: what limits growth rate? Biol Rev 86:97–116
Donohue K (2014) Why ontogeny matters during adaptation: developmental niche construction and pleitropy across the life cycle in Arabidopsis thaliana. Evolution 68:32–47
Dyrynda PEJ, King PE (1983) Gametogenesis in placental and non-placental ovicellate cheilostome bryozoa. J Zool 200:471–492
Dyrynda PEJ, Ryland JS (1982) Reproductive strategies and life histories in the cheilostome marine bryozoans Chartella papyracea and Bugula flabellata. Mar Biol 71:241–256
Edmunds PJ (2017) Intraspecific variation in growth rate is a poor predictor of fitness for reef corals. Ecology 98:2191–2200
Edmunds PJ, Putnam HM (2020) Science-based approach to using growth rate to assess coral performance and restoration outcomes. Biol Lett 16:20200227
Hart SP, Keough MJ (2009) Does size predict demographic fate? Modular demography and constraints on growth determine response to decreases in size. Ecology 90:1670–1678
Harvell CD, Helling R (1993) Experimental induction of localized reproduction in a marine bryozoan. Biol Bull 184:286–295
Hoffmann WA, Poorter H (2002) Avoiding bias in calculations of relative growth rate. Ann Bot 90:37–42
Hou C, Zuo W, Moses ME, Woodruff WH, Brown JH, West GB (2008) Energy uptake and allocation during ontogeny. Science 322:736–739
Hughes DJ, Hughes RN (1986) Metabolic implications of modularity: studies on the respiration and growth of Electra pilosa. Philos Trans R Soci B 313:23–29
Hughes TP, Jackson JBC (1980) Do corals lie about their age? Some demographic consequences of partial mortality, fission, and fusion. Science 209:713–715
Jackson JBC (1977) Competition on marine hard substrata: the adaptive significance of solitary and colonial strategies. Am Nat 111:743–767
Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7:1225–1241
Keough MJ (1986) The distribution of a bryozoan on seagrasses blades: settlement, growth and mortality. Ecology 67:846–857
Keough MJ (1989) Dispersal of the bryozoan Bugula neritina and effects of adults on newly metamorphosed juveniles. Mar Ecol Prog Ser 57:163–171
Keough MJ, Chernoff H (1987) Dispersal and population variation in the bryozoan Bugula neritina. Ecology 68:199–210
Kimball S, Gremer JR, Huxman TE, Lawrence Venable D, Angert AL (2013) Phenotypic selection favors missing trait combinations in coexisting annual plants. Am Nat 182:191–207
Kozłowski J, Uchmanski J (1987) Optimal individual growth and reproduction in perennial species with indeterminate growth. Evol Ecol 1:214–230
Madin JS, Hoogenboom MO, Connolly SR, Darling ES, Falster DS, Huang D, Keith SA, Mizerek T, Pandolfi JM, Putnam HM, Baird AH (2016) A trait-based approach to advance coral reef science. Trends Ecol Evol 31:419–428
Mangel M, Munch SB (2005) A life-history perspective on short- and long-term consequences of compensatory growth. Am Nat 166:E155–E176
Marshall DJ (2008) Transgenerational plasticity in the sea: context-dependent maternal effects across the life history. Ecology 89:418–427
Marshall DJ, White CR (2019) Have we outgrown the existing models of growth? Trends Ecol Evol 34:102–111
Mathew M, Schwaha T, Ostrovsky AN, Lopanik NB (2018) Symbiont-dependent sexual reproduction in marine colonial invertebrate: morphological and molecular evidence. Mar Biol 165:14
Monro K, Marshall DJ (2014) Faster is not always better: selection on growth rate fluctuates across life history and environments. Am Nat 183:798–809
Muggeo VMR, Atkins DC, Gallop RJ, Dimidjian S (2014) Segmented mixed models with random change points: a maximum likelihood approach with application to treatment for depression study. Stat Model 14:293–313
Ostrovsky AN (2013) Evolution of sexual reproduction in marine invertebrates: example of gymnolaemate bryozoans. Springer, Berlin, p 356
Pettersen AK, White CR, Marshall DJ (2015) Why does offspring size affect performance? Integrating metabolic scaling with life-history theory. Proc R Soc B 282:20151946
Pettersen AK, White CR, Marshall DJ (2016) Metabolic rate covaries with fitness and the pace of the life history in the field. Proc R Soc B 283:20160323
Pettersen AK, Hall MD, White CR, Marshall DJ (2020) Metabolic rate, context-dependent selection, and the competition-colonization trade-off. Evol Lett 4:333–344
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Sánchez JA, Lasker HR (2004) Do multi-branched colonial organisms exceed normal growth after partial mortality? Proc R Soc Lond 271:S117–S120
Schuster L, White CR, Marshall DJ (2019) Influence of food, body size, and fragmentation on metabolic rate in a sessile marine invertebrate. Invertebr Biol 138:55–66
Sebens KP (1987) The ecology of indeterminate growth in animals. Annu Rev Ecol Syst 18:371–407
Shaw RG, Geyer CJ, Wagenius S, Hangelbroek HH, Etterson JR (2008) Unifying life-history analyses for inference of fitness and population growth. Am Nat 172:E35–E47
Silén L (1977) Polymorphism. In: Woollacott RM, Zimmer RK (eds) Biology of bryozoans. Academic Press Inc, New York, pp 184–232
Ström R (1977) Brooding patterns of bryozoans. In: Woollacott RM, Zimmer RL (eds) Biology of bryozoans. Academic Press, London, pp 23–55
Teuschl Y, Reim C, Blackenhorn W (2007) Correlated responses to artificial body size selection in growth, development, phenotypic plasticity and juvenile viability in yellow dung flies. J Evol Biol 20:87–103
von Bertalanffy L (1957) Quantitative laws in metabolism and growth. Q Rev Biol 32:217–231
White CR, Marshall DJ (2019) Should we care if models are phenomenological or mechanistic? Trends Ecol Evol 34:276–278
Woollacott RM, Zimmer RK (1975) A simplified placenta-like system for the transport of extraembryonic nutrients during embryogenesis of Bugula neritina (Bryozoa). J Morphol 147:355–378
Zeileis A, Kleiber C, Jackman S (2008) Regression Models for Count Data in R. J. Stat. Software, Artic. 27:1–25
Acknowledgements
We thank two reviewers for comments that improved the manuscript. We thank the Florida State University (FSU) Coastal and Marine Laboratory for facilitating field work. This work was funded by grants to S. Burgess from FSU, including the Council on Research and Creativity in the FSU Office of Research. M. Bueno was supported by a grant from the São Paulo Research Foundation (FAPESP) (2016/23206‐1). Samples were collected under a Florida Fish and Wildlife Conservation Commission Special Activity License.
Funding
This work was funded by grants to S. Burgess from FSU, including the Council on Research and Creativity in the FSU Office of Research. M. Bueno was supported by a grant from the São Paulo Research Foundation (FAPESP) (2016/23206‐1).
Author information
Authors and Affiliations
Contributions
SCB conceived the project, analyzed the data, and wrote the paper. MB collected the data and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Samples were collected under a Florida Fish and Wildlife Conservation Commission Special Activity License.
Consent for publication
All authors consent to publication.
Additional information
Communicated by F. Bulleri.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by D. Denley and G. Ghedini.
Rights and permissions
About this article
Cite this article
Burgess, S.C., Bueno, M. When does growth rate influence fitness in a colonial marine invertebrate?. Mar Biol 168, 5 (2021). https://doi.org/10.1007/s00227-020-03804-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03804-9