Abstract
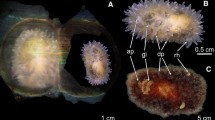
A series of cruises to the Mar del Plata Submarine Canyon (38°S/54°W) off Argentina in 2012–2013 have provided biological material that enables insights into the various modes of development of deep-sea invertebrates at depths up to 3500 m. This study describes the unusually large encapsulated embryos of the globose moon snail, Falsilunatia eltanini Dell, 1990 (Naticidae), and compares them with another direct-developing naticid from the same collections, Bulbus carcellesi. Embryos of F. eltanini develop in sand ribbon egg masses that contain up to 6 conspicuous egg capsules, 5.0–8.5 mm diameter. Each F. eltanini egg capsule contains a single, ~ 170-µm diameter egg and abundant, white, supplementary food. This allows the crawling pre-hatching juveniles to grow to 4.7 mm shell diameter. Different stages of development were found among multiple egg collars collected on the same date, which suggests a long reproductive season that could be continuous or periodic (lasting more than a year). The number of whorls in the hatchling juvenile shells and the significant size they attain confirm the occurrence of a long period of embryonic development. This reproductive strategy requires a large maternal investment in the very large egg capsules and abundant supplementary food. Within Naticidae, this extraordinary modality is only observed in several species inhabiting deep-sea and boreal cold waters.




Similar content being viewed by others
References
Amio M (1963) A comparative embryology of marine gastropods, with ecological considerations. J Shimonoseki Univ Fish 12:229–358
Ankel W (1930) Nähreierbildung bei Natica catena (da Costa). Zool Anz 89:129–135
Baxter R (1987) Mollusks of Alaska, a listing of all mollusks, freshwater, terrestrial, and marine reported from the State of Alaska, with locations of the species types, maximum sizes and marine depths inhabited. Shells and Sea Life, California
Berecoechea JJ, Brogger MI, Penchaszadeh PE (2017) New evidence of brooding in the deep-sea brittle star Astrotoma agassizii Lyman, 1876 from a South Western Atlantic Canyon. Deep-Sea Res I 127:105–110. https://doi.org/10.1016/j.dsr.2017.08.007
Bouchet P, Warén A (1993) Revision of the Northeast Atlantic bathyal and abyssal Mesogastropoda. Boll Malacol Suppl 3:577–840
Brandt A, Brökeland W, Brix S, Malyutina M (2004) Diversity of Southern Ocean deep-sea Isopoda (Crustacea, Malacostraca)—a comparison with shelf data. Deep-Sea Res II 51:1753–1768
Childress J, Price M (1978) Growth rate of the bathypelagic crustacean Gnathophausia ingens (Mysidacea: Lophogastridae). I. Dimensional growth and population structure. Mar Biol 50:47–62
Clarke A (1992) Reproduction in the cold: thorson revisited. Invertebr Reprod Dev 22:175–184
Colman J, Tyler P (1988) Observations on the reproductive biology of the deep-sea trochid Calliotropis ottoi (Philippi). J Molluscan Stud 54:239–242
Colman J, Tyler P, Gage J (1986) The reproductive biology of Colus jeffreysianus (Gastropoda: Prosobranchia) from 2200 m in the NE Atlantic. J Molluscan Stud 52:45–54
Dell RK (1990) Antarctic Mollusca: with special reference to the fauna of the Ross Sea. Bull R Soc NZ 27:1–311
Engl W (2012) Shells of Antarctica. ConchBooks, Hackenheim
Fioroni P (1982) Larval organs, larvae, metamorphosis and types of development of Mollusca: a comprehensive review. Zool Jb Abt Anat Ontogenie Tiere 108:375–420
Gallardo CS, Penchaszadeh PE (2001) Hatching mode and latitude in marine gastropods: revisiting Thorson’s paradigm in the southern hemisphere. Mar Biol 138:547–552
Giese AC, Kanatani H (1987) Maturation and spawning. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates. Blackwell Scientific Publication and The Boxwood Press, California, pp 252–313
Giglioli MEC (1955) The egg masses of the Naticidae (Gastropoda). J Fish Res Board Can 12:287–327
Gohar HAF, Eisawy AM (1967) The egg masses of five Rachiglossan prosobranchs from the Red Sea. Publ Mar Biol Stn Al-Ghardaqa 14:216–268
Hain S (1990) Die beschalten benthischen Mollusken (Gastropoda und Bivalvia) des Weddellmeeres, Antarktis = The benthic seashells (Gastropoda and Bivalvia) of the Weddell Sea, Antarctica. Ber Polarforsch 70:1–184
Hain S, Arnaud PM (1992) Notes on the reproduction of high-Antarctic molluscs from the Weddell Sea. Polar Biol 12:303–312
Hertling H (1932) Zur Kenntnis des Laichbandes und der Veligerlarven von Natica pulchella Risso. Zool Anz 100:95–100
Huelsken T, Marek C, Schreiber S, Schmidt I, Hollmann M (2008) The Naticidae (Mollusca: Gastropoda) of Giglio Island (Tuscany, Italy): Shell characters, live animals, and a molecular analysis of egg masses. Zootaxa 1770:1–40
Kohn AJ (2012) Egg size, life history, and tropical marine gastropod biogeography. Am Malacol Bull 30:163–174
Lardies MA, Fernández M (2002) Effect of oxygen availability in determining clutch size in Acanthina monodon. Mar Ecol Progr Ser 239:139–146
Lauretta D, Penchaszadeh PE (2017) Gigantic oocytes in the deep sea black coral Dendrobathypathes grandis (Antipatharia) from the Mar del Plata submarine canyon area (southwestern Atlantic). Deep-Sea Res I 128:109–114. https://doi.org/10.1016/j.dsr.2017.08.011
Lebour MV (1936) Notes on the eggs and larvae of some Plymouth prosobranchs. J Mar Biol Assoc UK 20:547–565
Levitan DR (2000) Optimal egg size in marine invertebrates: theory and phylogenetic analysis of the critical relationship between egg size and development time in echinoids. Am Nat 156:175–192
Martinez MI, Penchaszadeh PE (2017) A new species of brooding Psolidae (Echinodermata: Holothuroidea) from deep-sea off Argentina. Deep-Sea Res II, Southwestern Atlantic Ocean. https://doi.org/10.1016/j.dsr1012.2017.1005.1007
Montgomery EM, Hamel J-F, Mercier A (2016) The deep-sea neogastropod Buccinum scalariforme: reproduction, development and growth. Deep-Sea Res I 119:24–33
Moran AL (1999) Size and performance of juvenile marine invertebrates potential contrasts between intertidal and subtidal benthic habitats. Am Zool 39:304–312
Moran AL, Emlet RB (2001) Offspring size and performance in variable environments: field studies on a marine snail. Ecology 82:1597–1612
Murray F (1966) A brief account of the spawn of Conuber incei (Philippi, 1853). J Malacol Soc Australia 10:49–52
Natarajan AV (1957) Studies on the egg masses and larval development of some prosobranchs from the Gulf of Mannar and the Palk bay. Proc Indian Acad Sci 46:170–228
Pastorino G (2005) Recent Naticidae (Mollusca: Gastropoda) from the Patagonian Coast. Veliger 47:225–258
Pastorino G (2016) Revision of the genera Pareuthria Strebel, 1905, Glypteuthria Strebel, 1905 and Meteuthria Thiele, 1912 (Gastropoda: Buccinulidae) with the description of three new genera and two new species from Southwestern Atlantic waters. Zootaxa 4179:301–344
Pastorino G, Averbuj A, Penchaszadeh PE (2009) On the egg masses, eggs and embryos of Notocochlis isabelleana (d’Orbigny, 1840) (Gastropoda: Naticidae) from northern Patagonia. Malacologia 51:395–402
Pearse JS, Lockhart SJ (2004) Reproduction in cold water: paradigm changes in the 20th century and a role for cidaroid sea urchins. Deep-Sea Res II 51:1533–1549
Pedersen R, Page L (2000) Development and metamorphosis of the planktotrophic larvae of the moon snail, Polinices lewisii (Gould, 1847)(Caenogastropoda: Naticoidea). Veliger 43:58–63
Penchaszadeh P (1988) Reproductive patterns of some South American Prosobranchia as a contribution to classification. Malacol rev 4:284–287
Penchaszadeh PE, Atencio M, Martinez MI, Pastorino G (2016) Giant egg capsules and hatchlings in a deep-sea moon snail (Naticidae) from a southwestern Atlantic Canyon. Mar Biol 163:209. https://doi.org/10.1007/s00227-016-2990-z
Penchaszadeh PE, Teso V, Pastorino G (2017) Spawn in two deep-sea volute gastropods (Neogastropoda: Volutidae) from southwestern Atlantic waters. Deep-Sea Res I 130:55–62. https://doi.org/10.1016/j.dsr.2017.10.011
Perry JC, Roitberg BD (2006) Trophic egg laying: hypotheses and tests. Oikos 112:706–714
Rex M, Van Ummersen C, Turner R (1979) Reproductive pattern in the abyssal snail Benthonella tenella (Jeffreys). In: Stancyk SE (ed) Reproductive ecology of marine invertebrates. University of South Carolina Press, South Carolina, pp 173–188
Rivadeneira PR, Brogger MI, Penchaszadeh PE (2017) Aboral brooding in the deep water sea star Ctenodiscus australis Lütken, 1871 (Asteroidea) from the Southwestern Atlantic. Deep-Sea Res I 123:105–109
Rivest BR (1986) Extra-embryonic nutrition in the prosobranch gastropod Urosalpinx cinerea (Say, 1822). Bull Mar Sci 39:498–505
Robison B, Seibel B, Drazen J (2014) Deep-sea octopus (Graneledone boreopacifica) conducts the longest-known egg-brooding period of any animal. PLoS One 9:e103437
Rokop FJ (1974) Reproductive patterns in the deep-sea benthos. Science 186:743–745
Scheltema RS (1994) Adaptations for reproduction among deep-sea benthic molluscs: an appraisal. In: Young CM, Eckelbarger KJ (eds) Reproduction, larval biology, and recruitment of the deep-sea benthos. Columbia University Press, New York, pp 45–75
Spight TM (1976) Ecology of hatching size for marine snails. Oecologia 24:283–294
Thorson G (1935) Studies on the egg-capsules and development of Arctic marine prosobranchs. Medd Grønl 100(5):1–71
Thorson G (1936) The larval development, growth and metabolism of Antarctic marine bottom invertebrates. Medd Grønl 100(6):1–155
Thorson G (1940) Studies on the egg masses and larval development of gastropoda from the Iranian Gulf. Danish Sci Invest Iran 2:159–238
Thorson G (1946) Reproduction and larval development of Danish marine bottom invertebrates, with special reference to the planktonic larvae in the sound (Øresund). Medd Dan Fisk Havunders (Ser Plankton) 4:1–523
Thorson G (1950) Reproductive and larval ecology of marine bottom invertebrates. Biol Rev Camb Philos Soc 25:1–45
Torigoe K, Inaba A (2011) Revision on the classification of Recent Naticidae. Bull Nishinomiya Shell Mus 7:1–133
Tyler P, Young C (1992) Reproduction in marine invertebrates in “stable” environments: the deep sea model. Invertebr Reprod Dev 22:185–192
Tyler P, Grant A, Pain S, Gage J (1982) Is annual reproduction in deep-sea echinoderms a response to variability in their environment? Nature 300:747–750
Young CM (2003) Reproduction, development and life-history traits. In: Tyler PA (ed) Ecosystems of the deep-oceans. Elsevier, Amsterdam, pp 381–426
Acknowledgments
Special thanks are due to Alan Kabat for his thoughtful suggestions that highly improved the manuscript, to the Editors and to Juan Pablo Livore for reviewing the final manuscript. We thank Melina Atencio and Valeria Teso and the people involved in the ‘Talud Continental’ expeditions. This work was funded by PICT 2013–2504 from Agencia Nacional de Promoción Científica y Tecnológica, and PIP 0253 from Consejo Nacional de Investigaciones Científicas y Técnicas. This is publication #99 of LARBIM.
Funding
The present project was partially supported by Fondo para la Investigación Científica y Tecnológica, Argentina (FONCYT, PICT) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Andres Averbuj, Guido Pastorino and Pablo E. Penchaszadeh declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: J. Grassle.
Reviewed by A. Kabat.
Rights and permissions
About this article
Cite this article
Averbuj, A., Penchaszadeh, P.E. & Pastorino, G. Egg masses and development of Falsilunatia eltanini (Mollusca: Gastropoda): a deep-sea naticid from a Southwestern Atlantic Canyon. Mar Biol 165, 81 (2018). https://doi.org/10.1007/s00227-018-3337-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3337-8