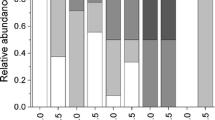
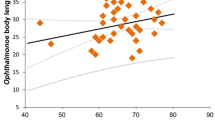
Abstract
The mating systems of symbiotic crustaceans vary broadly, ranging from monogamy to polygamy to promiscuity. This disparity in mating systems coupled with wide differences in host ecology provides opportunities to study the effect of environmental conditions on the social behavior of marine organisms. In this study, we tested the prediction that symbiotic crustaceans inhabiting relatively large and abundant host species are promiscuous rather than monogamous. The host traits above constraint host monopolization and favor host-switching behaviors by males individuals and, thus, ultimately drive promiscuity as a mating system in symbiotic crustaceans. As a model system we used Dissodactylus crinitichelis, a small ‘pea-crab’ that inhabits the relatively large and abundant sand dollar Encope emarginata. We described the population distribution of D. crinitichelis at two intertidal localities in Sergipe, Brazil, and conducted laboratory experiments to infer the mating system of this species. Dissodactylus crinitichelis inhabit sand dollars as adult heterosexual pairs more frequently than expected by chance alone, but these heterosexual pairs did not exhibit size-assortative pairing. Sexual dimorphism was reversed in D. crinitichelis, with males attaining smaller average body sizes than females but exhibiting more elaborated weaponry (male > female claws). These findings disagree with expectations for monogamous symbiotic crustaceans. Laboratory experiments demonstrated that male and female crabs switch among host individuals rather frequently and heterosexual pairs retrieved from the field did not remain together for long times. Our results argue against social monogamy and in favor of promiscuity in D. crinitichelis. Our study supports the notion that host abundance and large body size (relative to that of symbiotic guests) favor promiscuity in symbiotic crustaceans.







Similar content being viewed by others
References
Adams J, Edwards AJ, Emberton H (1985) Sexual size dimorphism and assortative mating in the obligate coral commensal Trapezia ferruginea Latreille (Decapoda, Xanthidae). Crustaceana 48:188–194
Ambrosio LJ, Baeza JA (2016) Territoriality and conflict avoidance explain a sociality (solitariness) of the endosymbiotic pea crab Tunicotheres moseri. PLoS One. doi:10.1371/journal.pone.0148285
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Baeza JA (1999) Indicators of monogamy in the commensal crab Pinnixa transversalis (Milne Edwards & Lucas) (Decapoda: Brachyura: Pinnoteridae): population distribution, male-female association and sexual dimorphism. Rev Biol Mar Oceanogr 34:303–313
Baeza JA (2008) Social monogamy in the shrimp Pontonia margarita, a symbiont of Pinctada mazatlantica, in the tropical eastern Pacific coast. Mar Biol 153:387–395
Baeza JA (2015) Crustaceans as symbionts: an overview of their diversity, host use and life styles. In: Watling L, Thiel M (eds) The life styles and feeding biology of the Crustacea. Oxford University Press, Oxford, pp 163–189
Baeza JA, Asorey CM (2012) Testing the role of male–male competition in the evolution of sexual dimorphism: a comparison between two species of porcelain crabs. Biol J Linn Soc Lond 105:548–558
Baeza JA, Hernáez P (2015) Population distribution, sexual dimorphism, and reproductive parameters in the crab Pinnixa valdiviensis Rathbun, 1907 (Decapoda: Pinnotheridae). J Crustac Biol 35:68–75
Baeza JA, Piantoni C (2010) Sexual system, sex ratio and group living in the shrimp Thor amboinensis (De Man): relevance to resource-monopolization and sex-allocation theories. Biol Bull 219:151–165
Baeza JA, Stotz W (2003) Host-use and selection of differently colored sea anemones by the symbiotic crab Allopetrolisthes spinifrons. J Exp Mar Biol Ecol 284:25–39
Baeza JA, Thiel M (2000) Host use pattern and life history of Liopetrolisthes mitra, a crab associate of the black sea urchin Tetrapygus niger. J Mar Biol Ass UK 80:639–645
Baeza JA, Thiel M (2003) Predicting territorial behavior in symbiotic crabs using host characteristics: a comparative study and proposal of a model. Mar Biol 142:93–100
Baeza JA, Thiel M (2007) The mating system of symbiotic crustaceans. A conceptual model based on optimality and ecological constraints. In: Duffy JE, Thiel M (eds) Reproductive and social behavior: Crustaceans as model systems. Oxford University Press, Oxford, pp 245–255
Baeza JA, Ritson-Williams R, Fuentes MS (2013) Sexual and mating system in a caridean shrimp symbiotic with the winged pearl oyster in the coral triangle. J Zool 289:172–181
Baeza JA, Hemphill CA, Ritson-Williams R (2015) The sexual and mating system of the shrimp Odontonia katoi (Palaemonidae, Pontoniinae), a symbiotic guest of the ascidian Polycarpa aurata in the coral triangle. PLoS One. doi:10.1371/journal.pone.0121120
Baeza JA, Simpson L, Ambrosio LJ, Guéron R, Mora N (2016) Monogamy in a hyper-symbiotic shrimp. PLoS One. doi:10.1371/journal.pone.0149797
Bauer RT (2004) Remarkable shrimps. Oklahoma University Press, Norman
Bauer RT, Thiel M (2011) First description of a pure-search mating system and protandry in the shrimp Rhynchocinetes uritai (Decapoda: Caridea). J Crustac Biol 31(2):286–295
Bell JL (1984) Changing residence: dynamics of the symbiotic relationship between Dissodactylus mellitae Rathbun (Pinnotheridae) and Mellita quinquiesperforata (Leske) (Echinodermata). J Exp Mar Biol Ecol 82:101–115
Campos E, Campos AR, León-González JA (2009) Diversity and ecological remarks of ectocommensals and ectoparasites (Annelida, Crustacea, Mollusca) of echinoids (Echinoidea: Mellitidae) in the Sea of Cortez, Mexico. Parasitol Res 105:479–487
Carranza A, Domingo A, Verdi A, Forselledo R, Estrades A (2003) First report of an association between Planes cyaneus (Decapoda: Grapsidae) and loggerhead sea turtles in the Southwestern Atlantic Ocean. Mar Turtle Newsl 102:5–7
Correa C, Thiel M (2003) Mating systems in caridean shrimp (Decapoda: Caridea) and their evolutionary consequences for sexual dimorphism and reproductive biology. Rev Chil Hist Nat 76:187–203
Crawley MJ (2013) Statistical computing—an introduction to data analysis using s-plus. Wiley, London
De Bruyn C, Rigaud T, Bruno D, Ridder C (2009) Symbiosis between the pea crab Dissodactylus primitivus and its echinoid host Meoma ventricosa: potential consequences for the crab mating system. Mar Ecol Prog Ser 375:173–183
De Bruyn C, David B, Ridder C, Rigaud T (2010) Asymmetric exploitation of two echinoid host species by a parasitic pea crab and its consequences for the parasitic life cycle. Mar Ecol Prog Ser 398:183–191
De Bruyn C, David B, Motreuil S, Caulier G, Jossart Q, Rigaud T, De Ridder C (2016) Should I stay or should I go? Causes and dynamics of host desertion by a parasitic crab living on echinoids. Mar Ecol Prog Ser 546:163–171
Duffy JE (1996) Eusociality in a coral-reef shrimp. Nature 381:512–514
Duffy JE (2007) Ecology and evolution of eusociality in sponge-dwelling shrimp. In: Duffy JE, Thiel M (eds) Evolutionary ecology of social and sexual systems: crustaceans as model organisms. Oxford University Press, Oxford, pp 387–409
Elliott JM (1983) Some methods for the statistical analysis of samples of benthic invertebrates, 3rd edn. Freshwater Biological Association, Far Sawrey
Gherardi F (1991) Eco-ethological aspects of the symbiosis between the shrimp Athanas indicus (Coutière 1903) and the sea urchin Echinometra mathaei (de Blainville 1825). Trop Zool 4(1):107–128. doi:10.1080/03946975.1991.10539481
Gray IE, McCloskey LR, Weihe SC (1968) The commensal crab Dissodactylus mellitae and its reaction to sand dollar host-factor. J Elisha Mitchell Sci Soc 84:472–481
Grove MW, Woodin SA (1996) Conspecific recognition and host choice in a pea crab, Pinnixa chaetopterana (Brachyura: Pinnotheridae). Biol Bull 190:359–366
Guilherme PDB, Brustolin MC, Bueno ML (2015) Distribution patterns of ectosymbiont crabs and their sand dollar hosts in a subtropical estuarine sandflat. Rev Biol Trop 63(Suppl. 2):209–220
Hartnoll RG (1978) The determination of relative growth in Crustacea. Crustaceana 34(3):281–293
Hartnoll RG (1982) Growth. In: Bliss DE, Abele LG (eds) The biology of Crustacea, 2, embryology, morphology and genetics. Academic Press, New York, pp 111–196
Jennions MD (1997) Female promiscuity and genetic incompatibility. Trends Ecol Evol 12(7):251–253
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev 75:21–64
Jossart Q, Wattier RA, Kastally C, Aron S, David B, Ridder CD, Riguad T (2014) Genetic evidence confirms polygamous mating system in a crustacean parasite with multiple hosts. PLoS One. doi:10.1371/journal.pone.0090680
Jr Haefner PA (1990) Morphometry and size at maturity of Callinectes ornatus (Brachyura, Portunidae) in Bermuda. Bull Mar Sci 46:274–286
Kathiresan K, Bingham BL (2001) Biology of mangroves and mangrove ecosystems. Adv Mar Biol 40:81–251
Martin JW, Davis GE (2006) Historical trends in crustacean systematics. Crustaceana 79(11):1347–1368
Martinelli-Filho JE, Santos RB, Ribeiro CC (2014) Host selection, host-use pattern and competition in Dissodactylus crinitichelis and Clypeasterophilus stebbingi (Brachyura: Pinnotheridae). Symbiosis. doi:10.1007/s13199-014-0292-0
Nogata Y, Matsumura K (2005) Larval development and settlement of a whale barnacle. Biol Lett 2:92–93
Ocampo EH, Nuñez JD, Cledón M, Baeza JA (2012) Host-specific reproductive benefits, host selection behavior and host use pattern of the pinnotherid crab Calytraeotheres garthi. J Exp Mar Biol Ecol 429:36–46
Ory NC, Dudgeon Thiel M (2013) Host-use patterns and factors influencing the choice between anemone and urchin hosts by a caridean shrimp. J Exp Mar Biol Ecol 449:85–92
Pérez GHB, Serrato MB, Sanchez CMD (2012) Equinodermos del Caribe colombiano II: Echinoidea Y Holothuroidea. Serie de Publicaciones Especiales de Invemar, Santa Marta
Pfaller JB, Alfaro-Shigueto J, Giffoni B, Ishihara T, Mangel JC, Peckham SH, Bjorndal KA, Baeza JA (2014) Social monogamy in the crab Planes major, a facultative symbiont of loggerhead sea turtles. J Exp Mar Biol Ecol 461:124–132
Pires-Vanin AMS (2001) Identifying the components of ecological variation in a marine benthic megafauna. Rev bras oceanogr 49(1/2):29–38
Reeves MV, Brooks WR (2001) Host selection, chemical detection, and protection of the symbiotic pinnotherid crabs Dissodactylus crinitichelis and Clypeasterophilus rugatus associated with echinoderms. Symbiosis 30:239–256
Sainte-Marie B (2007) Sperm demand and allocation in decapod Crustaceans. In: Duffy JE, Thiel M (eds) Reproductive and social behavior: Crustaceans as model systems. Oxford University Press, Oxford, pp 191–210
Shuster SM, Wade MJ (2003) Mating systems and strategies. Princeton University Press, Princeton
Sokal RR, Rohlf FJ (1981) Biometry, 3rd edn. Freeman, San Francisco
Thiel M, Zander A, Baeza JA (2003) Movements of the symbiotic crab Liopetrolisthes mitra between its host sea urchin Tetrapygus niger. Bull Mar Sci 72:89–101
Wehrtmann IS (1990) Distribution and reproduction of Ambidexter panamense and Palaemonetes schmitti in Pacific Costa Rica (Crustacea, Decapoda). Rev Biol Trop 38:327–329
Williams AB (1984) Shrimps, lobsters, and crabs of the Atlantic coast of the eastern United States, Maine to Florida. Smithsonian Institution Press, Washington
Wirtz P, Melo GAS, Grave S (2009) Symbioses of the decapod crustaceans along the coast of Espírito Santo, Brazil. Mar Biodivers Rec 2:1–9
Yanagisawa Y, Hamaishi A (1986) Mate acquisition by a solitary crab Zebrida adamsii, a symbiont of the sea urchin. J Ethol 4:153–162
Zar JH (2010) Biostatistical analysis, 5th edn. Prentice-Hall, Upper Saddle River
Acknowledgements
The authors are grateful to Dr. Sinara Maria Moreira for her help during fieldwork and experimental observations and to Dr. Leandro de Sousa Souto for help with some of the statistical analyses. The authors are also grateful to the reviewers and Dr. Martin Thiel for providing valuable criticism that helped us to improve earlier versions of the present manuscript. All sampling in this study was conducted according to the applicable state and federal laws. Thanks to Sarah Steedman and John L. Ambrosio for correcting the English language in previous versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not funded by any grant.
Conflict of interest
Author Douglas Fernandes Rodrigues Alves declares that he has no conflict of interest. Author Gustavo Luis Hirose declares that he has no conflict of interest. Author Samara de P. Barros-Alves declares that she has no conflict of interest. Author J. Antonio Baeza declares that he has no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: M. Thiel.
Reviewed by S. M. Shuster and undisclosed experts.
Rights and permissions
About this article
Cite this article
Alves, D.F.R., Hirose, G.L., Barros-Alves, S.d. et al. The mating system of the symbiotic pea-crab Dissodactylus crinitichelis (Brachyura, Pinnotheridae): monogamy or promiscuity?. Mar Biol 164, 200 (2017). https://doi.org/10.1007/s00227-017-3234-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-017-3234-6