Abstract
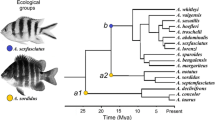
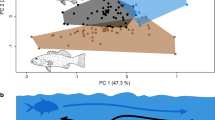
Coral and rocky reefs are productive and complex habitats that favor niche partitioning, the appearance of functional and morphological uniqueness in their inhabitants, and diversification. Members of the damselfish (Pomacentridae) subfamily Stegastinae, with 8 genera and 66 species, are among the most common inhabitants of reefs. Feeding behavior tends to be similar for most members of this subfamily, as they are benthic species that aggressively protects their feeding areas. Their main food sources are algae and invertebrates, but they also feed opportunistically on a wider range of resources. In this study, I analyzed the morphology of the pharyngeal jaw apparatus in an ecological and phylogenetic context to understand the role of these structures in promoting functional capacity and/or diversification. I found variation in the number and morphology of teeth, particularly in the lower pharyngeal jaw. The presence or absence of hard-shelled items in the diet appears to be a factor shaping the evolution of the pharyngeal jaw apparatus. Finally, the pharyngeal plates in the subfamily Stegastinae are highly diverse, reflecting specialization in processing particular prey items.





Similar content being viewed by others
References
Aguilar-Medrano R, Barber P (2016) Ecomorphological diversification in reef fish of the genus Abudefduf (Percifomes, Pomacentridae). Zoomorphology 135:103–114
Aguilar-Medrano R, Frédérich B, De Luna E, Balart EF (2011) Patterns of morphological evolution of the cephalic region in damselfishes (Perciformes, Pomacentridae) of the Eastern Pacific. Biol J Linn Soc 102:593–613
Aguilar-Medrano R, Frédérich B, Balart EF, De Luna E (2013) Diversification of the pectoral fin shape in damselfishes (Perciformes, Pomacentridae) of the Eastern Pacific. Zoomorphology 132:197–213
Aguilar-Medrano R, Kobelkowsky A, Balart EF (2015) Anatomical description of the Cortés damselfish Stegastes rectifraenum (Perciformes:Pomacentridae). Key structures for omnivore feeding. Rev Mex Biodivers 86:934–946
Allen GR (1991) Damselfishes of the world. Aquariums Systems, Mentor
Allen GR, Robertson DR (1994) Fishes of the Tropical Eastern Pacific. University of Hawaii Press, Honolulu
Allen GR, Woods LP (1980) A review of the damselfish genus Stegastes from the Eastern Pacific with description of a new species. Rec Aus Mus 8:171–198
Angel A, Ojeda PF (2001) Structure and trophic organization of subtidal fish assemblages on the northern Chilean coast: the effect of habitat complexity. Mar Ecol Prog Series 217:81–91
Barneche DR, Floeter SR, Ceccarelli DM, Frensel DMB, Dinslaken DF, Mário HFS (2009) Feeding macroecology of territorial damselfishes (Perciformes: Pomacentridae). Mar Biol 156:289–299
Baum DA, Allan L (1991) Adaptation reviewed: a phylogenetic methodology for studying character macroevolution. Syst Biol 40(1):1–18
Bazzaz FA (1975) Plant species diversity in old-field successional ecosystems in southern Illinois. Ecology 56(2):485–488
Bellwood DR, Choat JH (1990) A functional analysis of grazing in parrotfishes (family Scaridae): the ecological implications. Environ Biol Fish 28(1):189–214
Bookstein FL (1991) Morphometric tools for landmark data: geometry and biology. University Press, Cambridge
Burress ED, Duarte A, Serra WS, Loureiro M (2015) Rates of piscivory predict pharyngeal jaw morphology in a piscivorous lineage of cichlid fishes. Ecol Freshw Fish. doi:10.1111/eff.12236
Casciotta JR, Arratia G (1993) Jaws and teeth of American cichlids (Pisces: Labroidei). J Morphol 217:1–36
Cervigón F (1993) Los peces marinos de Venezuela, vol 2. Fundación Científica Los Roques, Caracas, Venezuela
Choat JH, Bellwood DR (1998) Wrasses and parrotfishes. In: Paxton JR, Eschmeyer WN (eds) Encyclopedia of animals:fishes. Academic Press, San Diego, pp 209–213
Ciardelli A (1967) The anatomy of the feeding mechanism and the food habits of Microspathodon chrysurus (Pisces: Pomacentridae). Bull Mar Sci 17:843–883
Cooper WJ, Smith LL, Weastneat MW (2009) Exploring the radiation of a diverse reef fish family: phylogenetics of the damselfishes (Pomacentridae), with new classifications based on molecular analyses of all genera. Mol Phyl Evol 52:1–16
Crowder LB, Cooper WE (1982) Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63(6):1802–1813
Davis JC (1986) Statistics and data analysis in geology. Wiley, New York
Dobzhansky T (1950) Evolution in the tropics. Am Sci 38:209–221
Dromard CR, Bouchon-Navaroa Y, Cordonniera S, Fontaineb M-F, Verlaqueb M, Harmelin-Vivienb M, Bouchona C (2013) Resource use of two damselfishes, Stegastes planifrons and Stegastes adustus, on Guadeloupean reefs (Lesser Antilles): inference from stomach content and stable isotope analysis. J Exp Mar Biol Ecol 440:116–125
Emery AR (1973) Comparative ecology and functional osteology of fourteen species of damselfish (Pisces: Pomacentridae) at Alligator Reef, Florida Keys. Bull Mar Sci 23:649–770
Eschmeyer WN, Fricke R, van der Laan R (2016) Catalog of fishes: genera, species, references. (http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp). Accessed Jun 2016
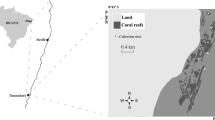
Espinoza M, Salas E (2005) Estructura de las comunidades de peces de arrecife en las Islas Catalinas y Playa Ocotal, Pacífico Norte de Costa Rica. Rev Biol Trop 53:523–536
Fraser RH, Currie DJ (1996) The species richness-energy hypothesis in a system where historical factors are thought to prevail: coral reefs. Am Nat 148(1):138–159
Frédérich B, Pilet A, Parmentier E, Vandewalle P (2008) Comparative trophic morphology in eight species of damselfishes (Pomacentridae). J Morphol 269:175–188
Frédérich B, Fabri G, Lepoint G, Vandewalle P, Parmentier E (2009) Trophic niches of thirteen damselfishes (Pomacentridae) at the Grand Recif of Toliara, Madagascar. Ichthyol Res 56:10–17
Frédérich B, Sorenson L, Santini F, Slater GJ, Alfaro ME (2013) Iterative ecological radiation and convergence during the evolutionary history of damselfishes (Pomacentridae). Am Nat 181:94–113
Frédérich B, Cooper WJ, Aguilar-Medrano R (2016) Ecomorphology and iterative ecological radiation of damselfishes. In: Frédérich B, Parmentier E (eds) Biology of damselfishes. CRC Press, Florida, pp 184–203
Froese R, Pauly D (2016) FishBase: concepts, design and data sources. ICLARM, Los Baños, Laguna. http://www.fishbase.org. Accessed May 2016
Galis F (2001) Key innovations and radiations. In: Wagner GP (ed) The character concept in evolutionary biology. Academic Press, San Diego, pp 581–605
Galis F, Drucker EG (1996) Pharyngeal biting mechanics in centrarchid and cichlid fishes: insights into a key evolutionary innovation. J Evol Biol 9:641–670
Gluckmann I, Vandewalle P (1998) Morphofunctional analysis of the feeding apparatus in four Pomacentridae species: Dascyllus aruanus, Chromis retrofasciata, Chrysiptera biocellata and C. unimaculata. Ital J Zool 65:421–424
Grove JG, Lavenberg RJ (1997) The fishes of the Galapagos Islands. Stanford University Press, Stanford, California
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: palentological statistics software package for education and data analysis. Palaeontolia Electronica 4(1):9. http://folk.uio.no/ahammer/past
Hellig CJ, Kerschbaumer M, Sefc KM, Koblmüller S (2010) Allometric shape change of the lower pharyngeal jaw correlates with a dietary shift to piscivory in a cichlid fish. Naturwissenschaften 97(7):663–672
Hixon MA (1981) An experimental analysis of territoriality in the California reef fish Embiotoca jacksoni (Embiotocidae). Copeia 1981:653–665
Hobson ES (1965) Diurnal-nocturnal activity of some inshore fishes in the Gulf of California. Copeia 1965:291–302
Hoogerhoud RJC (1986) The adverse effects of shell ingestion for molluscivorous cichlids, a constructional morphological approach. Neth J Zool 37(3):277–300
Hulsey CD (2006) Function of a key morphological innovation: fusion of the cichlid pharyngeal jaw. Proc R Soc B 273:669–675
Huysseune A (1995) Phenotypic plasticity in the lower pharyngeal jaw dentition of Astatoreochromis alluaudi (teleostei: cichlidae). Arch Oral Biol 40(11):1005–1014
Kaufman LS, Liem KF (1982) Fishes of the suborder Labroidei (Pisces: Perciformes): phylogeny, ecology and evolutionary significance. Breviora 472:1–19
Kuo SR, Shao KT (1991) Feeding habits of damselfish (Pomacentridae) from the southern part of Taiwan. J Fish Soc Taiwan 18(3):165–176
Liem KF (1973) Evolutionary strategies and morphological innovations: cichlid pharyngeal jaws. Syst Zool 22:425–441
Liem KF (1980) Adaptive significance of intra- and interspecific differences in the feeding repertoires of cichlid fishes. Am Zool 20:295–314
Liem KF, Sanderson SL (1986) The pharyngeal jaw apparatus of labrid fishes: a functional morphological perspective. J Morphol 187:143–158
Losos JB (2010) Adaptive radiation, ecological opportunity and evolutionary determinism. Am Nat 175(6):623–639
MacArthur RH, MacArthur JW (1961) On bird species diversity. Ecology 42(3):594–598
McGee MD, Borstein SR, Neches RY, Buescher HH, Seehausen O, Wainwright PC (2015) A pharyngeal jaw evolutionary innovation facilitated extinction in Lake Victoria cichlids. Science 350(6264):1077–1079
Montgomery WL (1980) Comparative feeding ecology of two herbivorous damselfishes (Pomacentridae: Teleostei) from the Gulf of California, Mexico. J Exp MarBiol Ecol 47:9–24
Moreno-Sánchez XG, Abitia-Cárdenas LA, Escobar-Sánchez O, Palacios-Salgado DS (2011) Diet of the Cortez damselfish Stegastes rectifraenum (Teleostei: Pomacentridae) from the rocky reef at Los Frailes, Baja California Sur, Mexico. Mar Biodivers Rec 4:e98. doi:10.1017/S1755267211000996
Muschick M, Barluenga M, Salburger W, Meyer A (2011) Adaptive phenotypic plasticity in the Midas cichlid fish pharyngeal jaw and its relevance in adaptive radiation. BMC Evol Biol 11:116
Pauly D, Froese R, Sa-a PS, Palomares ML, Christensen V, Rius J (2000) Trophlab manual. ICLARM, Manila
Petersen CW, Marchetti K (1989) Filial cannibalism in the Cortez Damselfish Stegastes rectifraenum. Evolution 43:58–168
Poisot T, Thrall PH, Hochberg ME (2012) Trophic network structure emerges through antagonistic coevolution in temporally varying environments. Proc R Soc B 279:299–308
Price NN, Hamilton SL, Smith JE, Tootell JS (2011) Species-specific consequences of ocean acidification for the calcareous tropical green algae Halimeda. Mar Ecol Prog Ser 440:67–78
R Development Core Team (2011) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. See http://R-project.org/
Randall JE (1996) Shore fishes of Hawai‘i. University of Hawai‘i Press, Honolulu
Reidenbach MA, Koseff JR, Koehl MAR (2009) Hydrodynamic forces on larvae affect their settlement on coral reefs in turbulent, wave-driven flow. Limnol Oceanogr 54(1):318–330
Revell LJ (2012) Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223
Revell LJ (2013) Two new graphical methods for mapping trait evolution on phylogenies. Methods Ecol Evol 4:754–759
Robertson DR, Allen GR (2008) Shorefishes of the Tropical Eastern Pacific online information system, Version 1.0. Smithsonian Tropical Research Institute, Balboa, Panamá. http://www.neotropicalfishes.org/sftep. http://www.stri.org/sftep
Rohlf FJ (1993) Relative warps analysis and an example of its application to mosquito wings. Contributions to morphometrics. In: Marcus LF, Bello E, Garcia-Valdecasas A (eds) Monografías del Museo Nacional de Ciencias Naturales. CSIC, Madrid, pp 139–151
Rohlf FJ. 2016. Tps series. Suny stonny brook. http://life.bio.sunysb.edu/morph/
Rohlf FJ, Slice D (1990) Extension of the Procrustes method for the optimal superposition of landmarks. Syst Zool 39:40–59
Schluter D (2000) Ecological character displacement in adaptive radiation. Am Nat 156:S4–S16
Schoener TW (1974) Resource partitioning in ecological communities. Science 185(4145):27–39
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Stiassny MLJ, Jensen JS (1987) Labroid intrarelationships revisited: morphological complexity, key innovations and the study of comparative diversity. Bull Mus Comp Zool 151:269–319
Thompson DA (1917) On growth and form. Cambridge University Press, Cambridge
Trapani J (2004) A morphometric analysis of polymorphism in the pharyngeal dentition of Cichlasoma minckleyi (Teleostei: Cichlidae). Arch Oral Biol 49(10):825–835
Vermeij GJ (1987) Evolution and escalation: an ecological history of life. Princeton University Press, Princeton
Vermeij GJ, Covich AP (1978) Coevolution of freshwater gastropods and their predators. Am Nat 112:833–843
Wainwright PC (1987) Biomechanical limits to ecological performance: mollusc crushing by the Caribbean hogfish, Lachnolaimus maximus (Labridae). J Zool Lond 213:283–298
Wainwright PC (1991) Ecomorphology: experimental functional anatomy for ecological problems. Am Zool 31:680–693
Wainwright PC, Bellwood DR (2002) Ecomorphology of feeding in coral reef fishes. In: Sale PF (ed) Coral reef fishes. Dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 33–55
Wainwright PC, Smith WL, Price SA, Tang KL, Sparks JS, Ferry LA, Kuhn KL, Eytan RI, Near TJ (2012) The evolution of pharyngognathy: a phylogenetic and functional appraisal of the pharyngeal jaw key innovation in labroid fishes and beyond. Syst Biol 61(6):1001–1027
Wilson S, Bellwood DR (1997) Cryptic dietary components of territorial damselfishes (Pomacentridae, Labroidei). Mar Ecol Prog Ser 153:299–310
Winemiller KO (1989) Ontogenetic diet shifts and resource partitioning among piscivorous fishes in the Venezuelan ilanos. Environ Biol Fish 26:177–199
Yashpal M, Kumari U, Mittal S, Mittal AK (2006) Surface architecture of the mouth cavity of a carnivorous fish Rita rita (Hamilton, 1822) (Siluriformes, Bagridae). Belg J Zool 136(2):155–162
Yoder JB, Clancey E, Des Roches S, Eastman JM, Gentry L, Godsoe W, Hagey TJ, Jochimsen D, Oswald BP, Robertson J, Sarver BAJ, Schenk JJ, Spear SF, Harmon LJ (2010) Ecological opportunity and the origin of adaptive radiations. J Evol Biol 23(8):1420–9101
Acknowledgements
I wish to thank the Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico, and the University of California Institute for Mexico and the United States (UC MEXUS), USA, which funded my postdoctoral research, and the Universidad Autónoma de Tamaulipas (Project PFI2015-06) for support of this project. Kevin McKeegan and Winnie Wu from the Department of Earth, Planetary and Space Sciences of University of California, Los Angeles, helped with the scanning electron microscopy. Lucia Campos (CIBNOR), H.J. Walker (SIO), Phil Hastings (SIO) and Rick Feeney (LACM) assisted in the fish collections. Edward Burress provided his data on cichlids. Three reviewers provided comments and revisions which greatly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Ethical standard
This study did not use live organisms.
Additional information
Responsible Editor: K.D. Clements.
Reviewed by L. Montgomery, E. Parmentier and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aguilar-Medrano, R. Ecomorphology and evolution of the pharyngeal apparatus of benthic damselfishes (Pomacentridae, subfamily Stegastinae). Mar Biol 164, 21 (2017). https://doi.org/10.1007/s00227-016-3051-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-3051-3