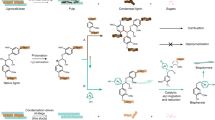
Abstract
The direct use of lignin results in the synthesis and production of low-value materials. Therefore, the chemical modification of lignin increases not only its reactivity, but also improves its compatibility and better dispersion in the lignin-based polymeric materials where lignin acts as a good substitute for oil-based polyols. Systematic studies were conducted in the present work. Hydroxymethylated lignin (HML) was synthesized via its reaction with formaldehyde, and functional groups present in the sol fraction of HML were then quantitatively investigated by aqueous potentiometric/conductometry titration prior to any further modification and by 1HNMR and FTIR techniques after reacting with α-bromoisobutyryl bromide, resulting in brominated HML (BHML). To calculate moles of hydroxyl groups per gram of HML, 1HNMR of BHML was recorded in the presence of a given amount of N,N-dimethylformamide as an internal standard. It was found that the number of hydroxyl groups increases from 6.44 mmol/g in the lignin to 7.77 mmol/g in the HML. The moles of phenolic hydroxyl and carboxyl groups per gram of HML were calculated using aqueous titration. It was found that the moles of phenolic hydroxyl groups in the HML decrease by 19.8% compared to lignin. Due to poor solubility of the HML in tetrahydrofuran, BHML was used in the GPC analysis, from which the number average molecular weight (\(\bar{M}_{\rm{n}}\)) of the HML was obtained after some calculations to be 1014 g/mol. It was observed that modification of lignin with formaldehyde increases not only its functional groups but also increases its molecular weight by creating methylene bridges between the two lignin molecules.










Similar content being viewed by others
References
Abdollahi M, Mousavian SA, Asadi A, Varamesh A (2017) Qualitative structural characterization of two lignin samples and quantitative determination of hydroxyl and methoxyl functional groups in kraft lignin via acetylation. Iran J Wood Pap Sci Res 32:369–381. https://doi.org/10.22092/ijwpr.2017.109402.1388
Abdollahi M, Bairami Habashi R, Mohsenpour M (2019) Poly(ε-caprolactone) chains grafted from lignin, hydroxymethylated lignin and silica/lignin hybrid macroinitiators: synthesis and characterization of lignin- based thermoplastic copolymers. Ind Crops Prod 130:547–557. https://doi.org/10.1016/j.indcrop.2019.01.012
Adler E, Hernestam S (1955) Estimation of phenolic hydroxyl groups in lignin. Acta Chem Scand 9:319–334
Agirre I, Garcia I, Requies J et al (2011) Glycerol acetals, kinetic study of the reaction between glycerol and formaldehyde. Biomass Bioenergy 35:3636–3642
Agirre I, Güemez MB, Ugarte A et al (2013) Glycerol acetals as diesel additives: kinetic study of the reaction between glycerol and acetaldehyde. Fuel Process Technol 116:182–188
Bairami Habashi R, Abdollahi M (2018) Functional groups and structural characterization of unmodified and functionalized lignin by titration, elemental analysis, 1H-NMR and FTIR techniques. Iran J Polym Sci Technol 30:405–418. https://doi.org/10.22063/JIPST.2017.1515(Persian)
Benar P, Gonçalves AR, Mandelli D, Schuchardt U (1999) Eucalyptus organosolv lignins: study of the hydroxymethylation and use in resols. Bioresour Technol 68:11–16
Buono P, Duval A, Verge P et al (2016) New insights on the chemical modification of lignin: acetylation versus silylation. ACS Sustain Chem Eng 4:5212–5222
Cateto CA, Barreiro MF, Rodrigues AE, Belgacem MN (2009) Optimization study of lignin oxypropylation in view of the preparation of polyurethane rigid foams. Ind Eng Chem Res 48:2583–2589
Chakar FS, Ragauskas AJ (2004) Review of current and future softwood kraft lignin process chemistry. Ind Crops Prod 20:131–141
Chen C-L (1992) Determination of carbonyl groups. In: Lin SY, Dence CW (eds) Methods in lignin chemistry. Springer, Berlin, pp 446–457
Chopade SP, Sharma MM (1997) Acetalization of ethylene glycol with formaldehyde using cation-exchange resins as catalysts: batch versus reactive distillation. React Funct Polym 34:37–45
Crestini C, Lange H, Sette M, Argyropoulos DS (2017) On the structure of softwood kraft lignin. Green Chem 19:4104–4121
Diao B, Zhang Z, Zhu J, Li J (2014) Biomass-based thermogelling copolymers consisting of lignin and grafted poly(N-isopropylacrylamide), poly(ethylene glycol), and poly(propylene glycol). RSC Adv 4:42996–43003. https://doi.org/10.1039/C4RA08673B
Doherty W, Halley P, Edye L et al (2007) Studies on polymers and composites from lignin and fiber derived from sugar cane. Polym Adv Technol 18:673–678
Gonçalves AR, Schuchardt U, Bianchi ML, Curvelo AAS (2000) Piassava fibers (Attalea funifera): NMR spectroscopy of their lignin. J Braz Chem Soc 11:491–494
Gosselink RJA, Abächerli A, Semke H et al (2004) Analytical protocols for characterisation of sulphur-free lignin. Ind Crops Prod 19:271–281
Granata A, Argyropoulos DS (1995) 2-Chloro-4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaphospholane, a reagent for the accurate determination of the uncondensed and condensed phenolic moieties in lignins. J Agric Food Chem 43:1538–1544
Hu L, Pan H, Zhou Y, Zhang M (2011) Methods to improve lignin’s reactivity as a phenol substitute and as replacement for other phenolic compounds: a brief review. BioResources 6:3515–3525
Kim YS, Kadla JF (2010) Preparation of a thermoresponsive lignin-based biomaterial through atom transfer radical polymerization. Biomacromol 11:981–988
Kim YS, Youe WJ, Kim SJ et al (2015) Preparation of a thermoplastic lignin-based biomaterial through atom transfer radical polymerization. J Wood Chem Technol 35:251–259. https://doi.org/10.1080/02773813.2014.937006
Kühnel I, Podschun J, Saake B, Lehnen R (2015) Synthesis of lignin polyols via oxyalkylation with propylene carbonate. Holzforschung 69:531–538
Lai Y-Z (1992) Determination of phenolic hydroxyl groups. In: Lin SY, Dence CW (eds) Methods in lignin chemistry. Springer, Berlin, pp 423–434
Laurichesse S, Avérous L (2013) Synthesis, thermal properties, rheological and mechanical behaviors of lignins-grafted-poly(ε-caprolactone). Polym (United Kingdom). https://doi.org/10.1016/j.polymer.2013.05.054
Laurichesse S, Averous L (2014) Chemical modification of lignins: towards biobased polymers. Prog Polym Sci 39:1266–1290
Liu X, Yin H, Zhang Z et al (2015a) Functionalization of lignin through ATRP grafting of poly(2-dimethylaminoethyl methacrylate) for gene delivery. Colloids Surf B Biointerfaces 125:230–237. https://doi.org/10.1016/j.colsurfb.2014.11.018
Liu X, Zong E, Jiang J et al (2015b) Preparation and characterization of Lignin-graft-poly (ε-caprolactone) copolymers based on lignocellulosic butanol residue. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2015.08.046
Ludwig CH, Sarkanen KV (1971) Lignins: occurrence, formation, structure and reactions. Wiley, New York
Malutan T, Nicu R, Popa VI (2007) Contribution to the study of hydroxymetylation reaction of alkali lignin. BioResources 3:13–20
Mansouri N-E, Salvadó J (2007) Analytical methods for determining functional groups in various technical lignins. Ind Crops Prod 26:116–124. https://doi.org/10.1016/j.indcrop.2007.02.006
Månsson P (1983) Quantitative determination of phenolic and total hydroxyl groups in lignins. Holzforsch-Int J Biol Chem Phys Technol Wood 37:143–146
Mazar A, Jemaa N, Wafa W et al (2018) In fluence of membrane filtration on extraction and characteristics of lignin from a kraft dissolving pulp mill pre-hydrolysate. Ind Crop Prod 124:726–734. https://doi.org/10.1016/j.indcrop.2018.08.046
Novikov N, Mozoleva AP, Uvarov AV et al (1986) Analysis of the 13C and 1H NMR spectra of phenol-formaldehyde resole oligomers. Polym Sci USSR 28:1187–1196
Perez-Camargo RA, Saenz G, Laurichesse S et al (2015) Nucleation, crystallization, and thermal fractionation of poly (ε-caprolactone)-grafted-lignin: effects of grafted chains length and lignin content. J Polym Sci, Part B: Polym Phys 53:1736–1750. https://doi.org/10.1002/polb.23897
Qiu X, Li H, Deng Y et al (2014) The acetylation of alkali lignin and its use for spherical micelles preparation. Acta Polym Sin 11:1458–1464
Serrano L, Esakkimuthu ES, Marlin N et al (2018) Fast, easy, and economical quantification of lignin phenolic hydroxyl groups: comparison with classical techniques. Energy Fuels 32:5969–5977. https://doi.org/10.1021/acs.energyfuels.8b00383
Stücker A, Podschun J, Saake B, Lehnen R (2018) A novel quantitative 31 P NMR spectroscopic analysis of hydroxyl groups in lignosulfonic acids. Anal Methods 10:3481–3488
Thring RW, Chornet E, Overend RP (1990) Recovery of a solvolytic lignin: effects of spent liquor/acid volume ratio, acid concentration and temperature. Biomass 23:289–305. https://doi.org/10.1016/0144-4565(90)90038-L
Wang H, Xue Y, Chen Y et al (2012) Lignin modification improves the biofuel production potential in transgenic Populus tomentosa. Ind Crops Prod 37:170–177
Yang C, Liu P (2009) Water-dispersed conductive polypyrroles doped with lignosulfonate and the weak temperature dependence of electrical conductivity. Ind Eng Chem Res 48:9498–9503
Yang L, Wang X, Cui Y et al (2014) Modification of renewable resources-lignin-by three chemical methods and its applications to polyurethane foams. Polym Adv Technol 25:1089–1098. https://doi.org/10.1002/pat.3356
Zhang J, Yu L, Wang Z et al (2011) Spherical microporous/mesoporous activated carbon from pulping black liquor. J Chem Technol Biotechnol 86:1177–1183
Zhou M, Huang K, Qiu X, Yang D (2012) Content determination of phenolic hydroxyl and carboxyl in lignin by aqueous phase potentiometric titration. CIESC J 63:258–265
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bairami Habashi, R., Abdollahi, M. Hydroxymethylation followed by α-bromoisobutyrylation as an effective and precise method for characterization of functional groups of hydroxymethylated lignin. Wood Sci Technol 54, 615–636 (2020). https://doi.org/10.1007/s00226-020-01176-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-020-01176-3