Abstract
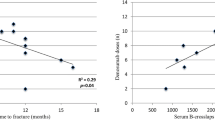
Bone-active drugs are recommended to protect the skeleton from detrimental actions of aromatase inhibitors (AIs). However, most of literature data are focused on bone mineral density (BMD), whereas data on fractures are scant. The aim of this prospective study was to investigate the real-life effectiveness of denosumab, oral bisphosphonates (BPs) and intravenous zoledronate on risk of vertebral fractures (VFs) induced by AIs. 567 consecutive women (median age 62 years, range 28–83) with early breast cancer undergoing treatment with AIs were evaluated for morphometric VFs and BMD at baseline and after 18–24 months of follow-up. After enrollment, 268 women (47.3%) started denosumab 60 mg subcutaneously every 6 months, 115 (20.3%) BPs (59 with oral BPs and, 56 with intravenous zoledronate 5 mg/12 months), whereas 184 women (32.5%) were not treated with bone-active drugs for several reasons. During follow-up, 54 women (9.5%) developed incident VFs in association with age of subjects (P < 0.001), baseline FRAX scores for major fractures (P < 0.001) and hip fractures (P = 0.003), pre-existing VFs (P < 0.001), change in BMD at lumbar spine (P = 0.015), femoral neck (P = 0.003) and total hip (P < 0.001). Risk of VFs was higher in subjects who were untreated as compared to those treated with bone-active drugs (32/184 vs. 22/383; P < 0.001). Specifically, fracture risk was significantly decreased by denosumab [odds ratio (OR) 0.22; P < 0.001] and zoledronate (OR 0.27; P = 0.035), but not by oral BPs (P = 0.317). These data suggest that in real-world clinical practice, denosumab and zoledronate can reduce AI-related risk of VFs after only 24 months of treatment.


Similar content being viewed by others
Data Availability
The datasets generated and analyzed during the current study are available in the ZENODO repository.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 71:209–249
Hadji P, Aapro MS, Body JJ, Gnant M, Brandi ML, Reginster JY, Zillikens MC, Glüer CC, de Villiers T, Baber R, Roodman GD, Cooper C, Langdahl B, Palacios S, Kanis J, Al-Daghri N, Nogues X, Eriksen EF, Kurth A, Rizzoli R, Coleman RE (2017) Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in postmenopausal women with hormone sensitive breast cancer: joint position statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol 7:1–12
(EBCTCG) EBCTCG (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet (London, England) 386:1341–1352
Mazziotti G, Canalis E, Giustina A (2010) Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med 123:877–884
Hadji P (2009) Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Crit Rev Oncol Hematol 69:73–82
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS (2005) Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet (London, England) 365:60–62
Lems WF, Paccou J, Zhang J, Fuggle NR, Chandran M, Harvey NC, Cooper C, Javaid K, Ferrari S, Akesson KE (2021) Vertebral fracture: epidemiology, impact and use of DXA vertebral fracture assessment in fracture liaison services. Osteoporos Int 32:399–411
Pedersini R, Monteverdi S, Mazziotti G, Amoroso V, Roca E, Maffezzoni F, Vassalli L, Rodella F, Formenti AM, Frara S, Maroldi R, Berruti A, Simoncini E, Giustina A (2017) Morphometric vertebral fractures in breast cancer patients treated with adjuvant aromatase inhibitor therapy: a cross-sectional study. Bone 97:147–152
Adachi JD, Adami S, Gehlbach S, Anderson FA Jr, Boonen S, Chapurlat RD, Compston JE, Cooper C, Delmas P, Díez-Pérez A, Greenspan SL, Hooven FH, LaCroix AZ, Lindsay R, Netelenbos JC, Wu O, Pfeilschifter J, Roux C, Saag KG, Sambrook PN, Silverman S, Siris ES, Nika G, Watts NB (2010) Impact of prevalent fractures on quality of life: baseline results from the global longitudinal study of osteoporosis in women. Mayo Clin Proc 85:806–813
Jalava T, Sarna S, Pylkkänen L, Mawer B, Kanis JA, Selby P, Davies M, Adams J, Francis RM, Robinson J, McCloskey E (2003) Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 18:1254–1260
Shapiro CL, Van Poznak C, Lacchetti C, Kirshner J, Eastell R, Gagel R, Smith S, Edwards BJ, Frank E, Lyman GH, Smith MR, Mhaskar R, Henderson T, Neuner J (2019) Management of osteoporosis in survivors of adult cancers with nonmetastatic disease: ASCO clinical practice guideline. J Clin Oncol 37:2916–2946
Miyashita H, Satoi S, Kuno T, Cruz C, Malamud S, Kim SM (2020) Bone modifying agents for bone loss in patients with aromatase inhibitor as adjuvant treatment for breast cancer; insights from a network meta-analysis. Breast Cancer Res Treat 181:279–289
Bassatne A, Bou Khalil A, Chakhtoura M, Arabi A, Van Poznak C, El-Hajj Fuleihan G (2021) Effect of antiresorptive therapy on aromatase inhibitor induced bone loss in postmenopausal women with early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Metabolism 128:154962
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England) 370:1453–1457
Rossini M, Adami S, Bertoldo F, Diacinti D, Gatti D, Giannini S, Giusti A, Malavolta N, Minisola S, Osella G, Pedrazzoni M, Sinigaglia L, Viapiana O, Isaia GC (2016) Guidelines for the diagnosis, prevention and management of osteoporosis. Reumatismo 68:1–39
Dalla Volta A, Mazziotti G, Maffezzoni F, Grisanti S, Palumbo C, Pedersini R, Maroldi R, Berruti A (2020) Bone mineral density and FRAX score may not predict fracture risk in patients with cancer undergoing hormone deprivation therapies. J Clin Oncol 38:3363–3366
Mazziotti G, Vena W, Pedersini R, Piccini S, Morenghi E, Cosentini D, Zucali P, Torrisi R, Sporeni S, Simoncini EL, Maroldi R, Balzarini L, Lania AG, Berruti A (2022) Prediction of vertebral fractures in cancer patients undergoing hormone deprivation therapies: reliability of who fracture risk assessment tool (frax) and bone mineral density in real-life clinical practice. J Bone Oncol 33:100421
Clark EM, Carter L, Gould VC, Morrison L, Tobias JH (2014) Vertebral fracture assessment (VFA) by lateral DXA scanning may be cost-effective when used as part of fracture liaison services or primary care screening. Osteoporos Int 25:953–964
Griffith JF, Genant HK (2012) New advances in imaging osteoporosis and its complications. Endocrine 42:39–51
Engelke K, Stampa B, Steiger P, Fuerst T, Genant HK (2019) Automated quantitative morphometry of vertebral heights on spinal radiographs: comparison of a clinical workflow tool with standard 6-point morphometry. Arch Osteoporos 14:18
Crans GG, Genant HK, Krege JH (2005) Prognostic utility of a semiquantitative spinal deformity index. Bone 37:175–179
Schousboe JT, Shepherd JA, Bilezikian JP, Baim S (2013) Executive summary of the 2013 international society for clinical densitometry position development conference on bone densitometry. J Clin Densitom 16:455–466
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E (2008) FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int 19:385–397
Lindsay R, Pack S, Li Z (2005) Longitudinal progression of fracture prevalence through a population of postmenopausal women with osteoporosis. Osteoporos Int 16:306–312
Mirza F, Canalis E (2015) Management of endocrine disease: secondary osteoporosis: pathophysiology and management. Eur J Endocrinol 173:R131-151
Pedersini R, Amoroso V, Maffezzoni F, Gallo F, Turla A, Monteverdi S, Ardine M, Ravanelli M, Vassalli L, Rodella F, Formenti AM, Dalla Volta A, Simoncini EL, Giustina A, Maroldi R, Berruti A (2019) Association of fat body mass with vertebral fractures in postmenopausal women with early breast cancer undergoing adjuvant aromatase inhibitor therapy. JAMA Netw Open 2:e1911080
Mazziotti G, Lania AG, Canalis E (2022) Skeletal disorders associated with the growth hormone-insulin-like growth factor 1 axis. Nat Rev Endocrinol. https://doi.org/10.1038/s41574-022-00649-8
Lu H, Lei X, Zhao H, Elting L, Siricilla M, Ursani MA, Giordano SH, Suarez-Almazor M (2021) Bone mineral density at the time of initiating aromatase inhibitor therapy is associated with decreased fractures in women with breast cancer. J Bone Miner Res 36:861–871
Confavreux CB, Fontana A, Guastalla JP, Munoz F, Brun J, Delmas PD (2007) Estrogen-dependent increase in bone turnover and bone loss in postmenopausal women with breast cancer treated with anastrozole. Prev Bisphosphonates Bone 41:346–352
Pineda-Moncusí M, Garcia-Giralt N, Diez-Perez A, Servitja S, Tusquets I, Prieto-Alhambra D, Nogués X (2020) Increased fracture risk in women treated with aromatase inhibitors versus tamoxifen: beneficial effect of bisphosphonates. J Bone Miner Res 35:291–297
Gnant M, Pfeiler G, Dubsky PC, Hubalek M, Greil R, Jakesz R, Wette V, Balic M, Haslbauer F, Melbinger E, Bjelic-Radisic V, Artner-Matuschek S, Fitzal F, Marth C, Sevelda P, Mlineritsch B, Steger GG, Manfreda D, Exner R, Egle D, Bergh J, Kainberger F, Talbot S, Warner D, Fesl C, Singer CF (2015) Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet (London, England) 386:433–443
Sun S, Wang F, Dou H, Zhang L, Li J (2016) Preventive effect of zoledronic acid on aromatase inhibitor-associated bone loss for postmenopausal breast cancer patients receiving adjuvant letrozole. Onco Targets Ther 9:6029–6036
Eidtmann H, de Boer R, Bundred N, Llombart-Cussac A, Davidson N, Neven P, von Minckwitz G, Miller J, Schenk N, Coleman R (2010) Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol 21:2188–2194
Monda V, Lupoli GA, Messina G, Peluso R, Panico A, Villano I, Salerno M, Sessa F, Marciello F, Moscatelli F, Valenzano A, Molino L, Lupoli R, Fonderico F, Tortora A, Pisano A, Ruberto M, Gabriella M, Cavaliere G, Trinchese G, Mollica MP, Cipolloni L, Cibelli G, Monda M, Lupoli G, Messina A (2017) Improvement of bone physiology and life quality due to association of risedronate and anastrozole. Front Pharmacol 8:632
Buch-Larsen K, Jørgensen NR, Jensen LT, Andersson M, Schwarz P (2021) Denosumab vs. zoledronic acid treatment in post-menopausal breast cancer: a 2-year prospective observational study. Scand J Clin Lab Invest 81:425–431
van de Glind EM, Willems HC, Eslami S, Abu-Hanna A, Lems WF, Hooft L, de Rooij SE, Black DM, van Munster BC (2016) Estimating the time to benefit for preventive drugs with the statistical process control method: an example with alendronate. Drugs Aging 33:347–353
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K, Almasri J, Farah W, Sarigianni M, Muthusamy K, Al Nofal A, Haydour Q, Wang Z, Murad MH (2019) Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab 104:1623–1630
Jacob L, Kostev K, Hadji P (2017) Prevalence of bisphosphonate therapy in women with breast cancer treated with aromatase inhibitors in Germany. Int J Clin Pharmacol Ther 55:394–396
Diacinti D, Del Fiacco R, Pisani D, Todde F, Cattaruzza MS, Diacinti D, Arima S, Romagnoli E, Pepe J, Cipriani C, Minisola S (2012) Diagnostic performance of vertebral fracture assessment by the lunar iDXA scanner compared to conventional radiography. Calcif Tissue Int 91:335–342
Szulc P (2018) Bone turnover: biology and assessment tools. Best practice & research. Clin Endocrinol Metab 32:725–738
Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Zillikens MC (2017) Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone 105:11–17
Osella G, Puglisi S, Alì A, Reimondo G, Terzolo M (2021) Multiple rebound-associated vertebral fractures after denosumab discontinuation: is prompt antiresorptive therapy always recommended, even when the risk of fracture seems low? A case report. Endocr Metab Immune Disord Drug Targets 21:2303–2306
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
GM and AGL had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. GM, WV, RP, AGL, AB: Concept and design. GM, WV, RP, DC, FC, SP, RT, ELS, AZ: Enrollment of patients. LB, DF, WV, GM: Radiological examinations. GM, WV, RP, AGL, AB: Acquisition, analysis or interpretation of data. RP, GM: Statistical analysis. GM, AB, AGL: Drafting the manuscript. GM, WV, RP, AZ, AGL, AB: Critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The Authors declare no competing non-financial interests but the following competing financial interests: Dr. Mazziotti received consultancy fees from Novartis, Ipsen, Eli Lilly and lecture fees from Amgen and Abiogen, outside the submitted work; Dr. Vena received grants from IBSA Pharmaceutical outside the submitted work; Dr. Torrisi received research grants from Pfizer, consultancy fees from MSD and lecture fees from Pfizer, Eli Lilly, EISAI and Genomic Health outside the submitted work; Dr. Zambelli received consultancy fees from Roche, Novartis, Pfizer, Eli Lilly & Co., AstraZeneca, Genomic Health; Dr. Lania received grants from Pfizer and consultancy fees from Ipsen, outside the submitted work; Dr. Berruti reports receiving grants and personal fees from Janssen Cilag, grants and personal fees from Astellas, and personal fees from Bayer outside the submitted work.
Human and animal rights and informed consent
All the procedures performed in the study were in accordance with the ethical standards of the Ethics Committees of IRCCS Humanitas Research Hospital, Rozzano-Milan, Italy, Spedali Civili Hspital of Brescia, Italy and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All the enrolled subjects gave their informed consent to use the clinical and biochemical data for research purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
223_2022_1011_MOESM2_ESM.tif
Supplementary file2 (TIF 113 KB) Supplementary Figure 1 Flow-chart of enrollment of 567 women with early breast cancer starting aromatase inhibitor (AI) therapy and then stratified to be treated or not with bone-active drugs
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mazziotti, G., Pedersini, R., Vena, W. et al. Real-World Effectiveness of Denosumab and Bisphosphonates on Risk of Vertebral Fractures in Women with Breast Cancer Undergoing Treatment with Aromatase Inhibitors. Calcif Tissue Int 111, 466–474 (2022). https://doi.org/10.1007/s00223-022-01011-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-01011-w