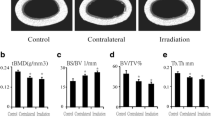
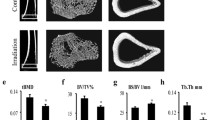
Abstract
Chronic and systemic bone complications frequently occur in patients who undergo radiotherapy; however, the pathological mechanisms underlying these complications remain unclear. This study aimed to observe persistent and systemic changes in locally irradiated rats and to determine the systemic pathological changes that persistently affect bone metabolism. We examined the inflammatory and oxidative stress responses that occurred after local irradiation using enzyme immunoassays and biochemical analyses. Lymphocytes obtained from the blood, spleen, thymus, and bone marrow were evaluated using flow cytometry. The proliferation and apoptosis characteristics of co-cultured bone marrow-derived mesenchymal stem cells (BMSCs) were detected by MTT assay and PI/Annexin V-FITC staining, respectively, and the differentiation of BMSCs was measured according to alkaline phosphatase (ALP) staining, alizarin red staining, and Oil Red O staining and by evaluating the mRNA expression of ALP, osteocalcin (OCN), osteopontin (OPN), collagen I, Runx2, and PPARγ. Our results revealed that no significant or continuous differences were present in the inflammatory response or the oxidative stress response throughout the body after local irradiation. B lymphocyte levels increased continuously in the blood, spleen, and bone marrow after local irradiation. T lymphocyte levels were decreased at 2 weeks after local irradiation, and CD8+T lymphocyte levels were increased in the blood, thymus, and bone marrow at 12 weeks after local irradiation. The ratio of CD4+/CD8+T lymphocytes began to decrease during the early phase after local irradiation and became significantly decreased at 12 weeks after local irradiation. Normal BMSCs co-cultured with lymphocytes derived from irradiated rats exhibited decreased proliferation and increased apoptosis, and the ALP staining intensity, alizarin red staining intensity, and mRNA expression of related genes were all also decreased. Oil Red O staining intensity and mRNA expression of PPARγ were both increased. Lymphocyte levels contribute to chronic and systemic bone complications after radiotherapy by inhibiting the proliferation and osteoblastogenesis of BMSCs.







Similar content being viewed by others
Abbreviations
- TNF-α:
-
Tumor necrosis factor-α
- IL-6:
-
Interleukin-6
- IFN-γ:
-
Interferon-γ
- IL-1α:
-
Interleukin-1α
- IL-1β:
-
Interleukin-1β
- MCP-1:
-
Monocyte chemoattractant protein 1
- MIP:
-
Macrophage inflammatory protein
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- MDA:
-
Malondialdehyde
- GSH-Px:
-
Glutathione peroxidase
- T-AOC:
-
Total antioxidant capacity
- BMSCs:
-
Bone marrow-derived mesenchymal stem cells
- RANKL:
-
Receptor activator of nuclear factor kappa B ligand
- BV/TV:
-
Ratio of the segmented bone volume to the total volume
References
Baldini EH, Le Cesne A, Trent JC (2018) Neoadjuvant chemotherapy, concurrent chemoradiation, and adjuvant chemotherapy for high-risk extremity soft tissue sarcoma. Am Soc Clin Oncol Educ Book 23(38):910–915. https://doi.org/10.1200/EDBK_201421
Heinrich S, Lang H (2017) Neoadjuvant therapy of pancreatic cancer: definitions and benefits. Int J Mol Sci. https://doi.org/10.3390/ijms18081622
D’Oronzo S, Stucci S, Tucci M, Silvestris F (2015) Cancer treatment-induced bone loss (CTIBL): pathogenesis and clinical implications. Cancer Treat Rev 41(9):798–808. https://doi.org/10.1016/j.ctrv.2015.09.003
Soares CBG, Araújo ID, Pádua BJ, Vilela JCS, Souza RHR, Teixeira LEM (2019) Pathological fracture after radiotherapy: systematic review of literature. Rev Assoc Med Bras 65(6):902–908. https://doi.org/10.1590/1806-9282.65.6.902
Kang YM, Chao TF, Wang TH, Hu YW (2019) Increased risk of pelvic fracture after radiotherapy in rectal cancer survivors: a propensity matched study. Cancer Med 8(8):3639–3647. https://doi.org/10.1002/cam4.2030
Chen Z, Maricic M, Aragaki AK, Mouton C, Arendell L, Lopez AM, Bassford T, Chlebowski RT (2009) Fracture risk increases after diagnosis of breast or other cancers in postemenopausal women: results from the Women’s Health Initiative. Osteoporos Int 20(4):527–536. https://doi.org/10.1007/s00198-008-0721-0
Pillai US, Kayal S, Cyriac S, Nisha Y, Dharanipragada K, Kamalanathan SK, Halanaik D, Kumar N, Madasamy P, Muniswamy DK, Dubashi B (2019) Late effects of breast cancer treatment and outcome after corrective interventions. Asian Pac J Cancer Prev 20(9):2673–2679. https://doi.org/10.31557/apjcp.2019.20.9.2673
Ramchand SK, Cheung YM, Grossmann M (2019) Bone health in women with breast cancer. Climacteric 22(6):589–595. https://doi.org/10.1080/13697137.2019.1580257
Jia D, Gaddy D, Suva LJ, Corry PM (2011) Rapid loss of bone mass and strength in mice after abdominal irradiation. Radiat Res 176(5):624–635. https://doi.org/10.1667/rr2505.1
Wright LE, BuijsJT Kim HS, Coats LE, Scheidler AM, John SK, She Y, Murthy S, Ma N, Chin-Sinex HJ, Bellido TM, Bateman TA, Mendonca MS, Mohammad KS, Guise TA (2015) Single-limb irradiation induces local and systemic bone loss in a murine model. J Bone Miner Res 30(7):1268–1279. https://doi.org/10.1002/jbmr.2458
Pan M, Hong W, Yao Y, Gao X, Zhou Y, Fu G, Li Y, Guo Q, Rao X, Tang P, Chen S, Jin W, Hua G, Gao J, Xu X (2019) Activated B lymphocyte inhibited the osteoblastogenesis of bone mesenchymal stem cells by notch signaling. Stem Cells Int. https://doi.org/10.1155/2019/8150123
Xu X, Li R, Zhou Y, Zou Z, Ding Q, Wang J, Jin W, Hua G, Gao J (2017) Dysregulated systemic lymphocytes affect the balance of osteogenic/daipogenic differentiation of bone mesenchymal stem cells after local irradiation. Stem Cell Res Ther 8(1):71. https://doi.org/10.1186/s13287-017-0527-0
Wissing MD (2015) Chemotherapy- and irradiation-induced bone loss in adults with solid tumors. Curr Osteoporos Rep 13(3):140–145. https://doi.org/10.1007/s11914-015-0266-z
Hayar M, Durankuş NK, Altun GD, Koçak Z, Uzal MC, Saynak M (2019) Investigation of differences of sacral and vertebral bone mineral densities before and after radiotherapy in patients with locally advanced rectal cancer. Cancer Radiother 23(5):408–415. https://doi.org/10.1016/j.canrad.2019.05.014
Bedatsova L, Drake MT (2019) The skeletal impact of cancer therapies. Br J Clin Pharmacol 85(6):1161–1168. https://doi.org/10.1111/bcp.13866
Tőkés T, Varga G, Garab D, Nagy Z, Fekete G, Tuboly E, Plangár I, Mán I, Szabó RE, Szabó Z, Volford G, Ghyczy M, Kaszaki J, Boros M, Hideghéty K (2014) Peripheral inflammatory activation after hippocampus irradiation in the rat. Int Radiat Biol 90(1):1–6. https://doi.org/10.3109/09553002.2013.836617
Cohen SR, Cohen EP (2013) Chronic oxidative stress after irradiation: an unproven hypothesis. Med Hypotheses 80(2):172–175. https://doi.org/10.1016/j.mehy.2012.11.022
Zou Q, Hong W, Zhou Y, Ding Q, Wang J, Jin W, Gao J, Hua G, Xu X (2016) Bone marrow stem cell dysfunction in radiation-induced abscopal bone loss. J Orthop Surg Res. https://doi.org/10.1186/s13018-015-0339-9
Walsh MC, Takegahara N, Kim H, Choi Y (2018) Updating osteoimmunology: regulation of bone cells by innate and adaptive immunity. Nat Rev Rheumatol 14(3):146–156. https://doi.org/10.1038/nrrheum.2017.213
Terashima A, Takayanagi H (2018) Overview of osteoimmunology. Calcif Tissue Int 102(5):503–511. https://doi.org/10.1007/s00223-018-0417-1
Azam Z, Anupam R, Mondal RK, Srivastava RK (2018) Osteoimmunology: the nexus between bone and immune system. Front Biosci (Landmark Ed) 23:464–492. https://doi.org/10.2741/4600
Srivastava RK, Dar HY, Mishra PK (2018) Immunoporosis: immunology of osteoporosis-role of T cells. Front Immunol 9:657. https://doi.org/10.3389/fimmu.2018.00657
Xu S, Zhang Y, Liu B, Li K, Huang B, Yan B, Zhang Z, Liang K, Jia C, Lin J, Zeng C, Cai D, Jin D, Jiang Y, Bai X (2016) Activation of mTORC1 in B lymphocytes promotes osteoclast formation via regulation of beta-catenin and RANKL/OPG. J Bone Miner Res 31(7):1320–1333. https://doi.org/10.1002/jbmr.2800
Weitzmann MN (2014) T-cells and B-cells in osteoporosis. Curr Opin Endocrinol Diabetes Obes 21(6):461–467. https://doi.org/10.1097/med.0000000000000103
Ryan MR, Shepherd R, Leavey JK, Gao Y, Grassi F, Schnell FJ, Qian WP, Kersh GJ, Weitzmann MN, Pacifici R (2005) An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Pro Natl Acad Sci USA 102(46):16735–16740. https://doi.org/10.1073/pnas.0505168102
Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, Weitzmann MN (2007) B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 109(9):3839–3848. https://doi.org/10.1182/blood-2006-07-037994
Yeo L, Toellner KM, Salmon M, Filer A, Buckley CD, Raza K, Scheel-Toellner D (2011) Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis 70(11):2022–2028. https://doi.org/10.1136/ard.2011.153312
Han X, Lin X, Seliger AR, Eastcott J, Kawai T, Taubman MA (2009) Expression of receptor activator of nuclear factor-kappa B ligand by B cells in response to oral bacteria. Oral Microbiol Immunol 24(3):190–196. https://doi.org/10.1111/j.1399-302x.2008.00494.x
Kanematsu M, Sato T, Takai H, Watanabe K, Ikeda K, Yamada Y (2000) Prostaglandin E-2 induces expression of receptor activator of nuclear factor B ligand/osteoprotegrin ligand on pre-B cells: implications for accelerated osteoclastogenesis in estrogen deficiency. J Bone Miner Res 15(7):1321–1329. https://doi.org/10.1359/jbmr.2000.15.7.1321
Onal M, Xiong J, Chen X, Thostenson JD, Almeida M, Manolagas SC, O’Brien CA (2012) Receptor activator of nuclear factor Kb ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem 287(35):29851–29860. https://doi.org/10.1074/jbc.m112.377945
Yang GC, Xu YH, Chen HX, Wang XJ (2015) Acute lymphoblastic leukemia cells inhibit the differentiation of bone mesenchymal stem cells into osteoblastsin vitro by activating notch signaling. Stem Cells Int 2015:162410. https://doi.org/10.1155/2015/162410
Shao B, Fu X, Yu Y, Yang D (2018) Regulatory effects of miRNA-181a on FasL expression in bone marrow mesenchymal stem cells and its effect on CD4+T lymphocyte apoptosis in estrogen deficiency-induced osteoporosis. Mol Med Rep 18(1):920–930. https://doi.org/10.3892/mmr.2018.9026
Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R (2001) Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA 98(24):13960–5. https://doi.org/10.1073/pnas.251534698
Acknowledgements
We acknowledge financial support from the Shanghai Municipal Health Commission (No.201740028) and National Natural Science Foundation of China (No.81102071).
Author information
Authors and Affiliations
Contributions
XX and WH designed the study and did the cell culture and answered all the questions of the reviewers; LT and XX performed the ELISA and flow cytometry experiments; WL, RG and LH contributed to the animal experiments; XX and XS analyzed the data; and WH wrote the manuscript. All authors have read and approved the final manuscript to be published.
Corresponding authors
Ethics declarations
Conflict of interest
Wei Hong, Lichen Tang, Rui Ge, Weiping Li, Xiaoyong Shen, Lixia Hong and Xiaoya Xu declare that they have no conflict of interest.
Ethical Approval
Ethical approval was provided by the Committee on Animal Resources of Fudan University.
Informed Consent
For this type of study, no informed consent is required.
Human and Animal Rights
All procedures performed in studies involving animals were in accordance with the ethical standards of the Institutional Animal Ethics Committee of Fudan University at which the studies were conducted (NO.2012-03-FYS-GJJ-01).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hong, W., Tang, L., Ge, R. et al. Persistent Abnormal Immunocytes Induced Systemic Bone Loss in Locally Irradiated Rats. Calcif Tissue Int 109, 706–718 (2021). https://doi.org/10.1007/s00223-021-00883-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00883-8