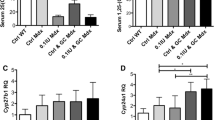
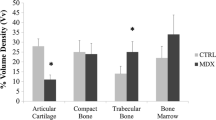
Abstract
Duchenne muscular dystrophy (DMD) is an X-linked disease of progressive muscle deterioration and weakness. Patients with DMD have poor bone health which is partly due to treatment with glucocorticoids, a standard therapy to prolong muscle function that also induces bone loss. Bisphosphonates are used to treat adults at risk of glucocorticoid-induced osteoporosis but are not currently used in DMD patients until after they sustain fractures. In this study, C57BL/10ScSn-mdx mice, a commonly used DMD animal model, received continuous glucocorticoid, prednisone treatment (0.083 mg/day) from 5 to 10 weeks of age. Pre-treatment with the bisphosphonate pamidronate started at 4 weeks of age over a period of 2 weeks or 6 weeks (cumulative dose 8 mg/kg for both) to assess the effectiveness of the two dosing regimens in ameliorating glucocorticoid-induced bone loss. Mdx mice treated with prednisone had improved muscle function that was not changed by pamidronate treatment. Glucocorticoid treatment caused cortical bone loss and decreased cortical bone strength. Both 2 and 6 week pamidronate treatment increased cortical thickness and bone area compared to prednisone-treated Mdx mice, however, only 2 week pamidronate treatment improved the strength of cortical bone compared to that of glucocorticoid-treated Mdx mice. In the trabecular bone, both pamidronate treatments significantly increased the amount of bone, and increased the ultimate load but not the energy to fail. These results highlight the importance of when and how much bisphosphonate is administered prior to glucocorticoid exposure.





Similar content being viewed by others
References
Emery AEH (1991) Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul Disord 1:19–29
Brooke MH, Fenichel GM, Griggs RC et al (1989) Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology 39:475–481
Mendell JR, Goemans N, Lowes LP et al (2016) Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann Neurol 79:257–271
Matthews E, Brassington R, Kuntzer T et al. (2016) Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst Rev 5:CD003725
Rufo A, Del Fattore A, Capulli M et al (2011) Mechanisms inducing low bone density in Duchenne muscular dystrophy in mice and humans. J Bone Miner Res 26:1891–1903
McDonald DG, Kinali M, Gallagher AC et al (2002) Fracture prevalence in Duchenne muscular dystrophy. Dev Med Child Neurol 44:695–698
Heutinck L, Kampen NV, Jansen M, Groot IJ (2017) Physical activity in boys with Duchenne muscular dystrophy is lower and less demanding compared to healthy boys. J Child Neurol 32:450–457
Morgenroth VH, Hache LP, Clemens PR (2012) Insights into bone health in Duchenne muscular dystrophy. BoneKEy Rep 1:1–11
Redlich K, Smolen JS (2012) Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov 11:234–250
Biggar WD, Harris VA, Eliasoph L, Alman B (2006) Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul Disord 16(4):249–255
Singh A, Schaeffer EK, Reilly CW (2018) Vertebral fractures in Duchenne muscular dystrophy patients managed with deflazacort. J Pediatr Orthop 38:320–324
Fazil M, Baboota S, Sahni JK et al (2015) Bisphosphonates: therapeutics potential and recent advances in drug delivery. Drug Deliv 22:1–9
Ward L, Tricco AC, Phuong P et al. (2007) Bisphosphonate therapy for children and adolescents with secondary osteoporosis. Cochrane Database Syst Rev 4:CD005324
Buckner JL, Bowden SA, Mahan JD (2015) Optimizing bone health in Duchenne muscular dystrophy. Int J Endocrinol 2015:928385
Hawker GA, Ridout R, Harris VA et al (2005) Alendronate in the treatment of low bone mass in steroid-treated boys with Duchennes muscular dystrophy. Arch Phys Med Rehabil 86:284–288
Gordon KE, Dooley JM, Sheppard KM,et al (2011) Impact of bisphosphonates on survival for patients with Duchenne muscular dystrophy. Pediatrics 127:e353–e358
Sbrocchi AM, Rauch F, Jacob P et al (2012) The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos Int 23:2703–2711
Luckman SP, Hughes DE, Coxon FP et al (1998) Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 13:581–589
Srinivasan R, Rawlings D, Wood CL et al (2016) Prophylactic oral bisphosphonate therapy in duchenne muscular dystrophy. Muscle Nerve 54:79–85
Yoon SH, Chen J, Grynpas MD, Mitchell J (2016) Prophylactic pamidronate partially protects from glucocorticoid-induced bone loss in the mdx mouse model of Duchenne muscular dystrophy. Bone 90:168–180
Yoon SH, Sugamori KS, Grynpas MD, Mitchell J (2016) Positive effects of bisphosphonates on bone and muscle in a mouse model of Duchenne muscular dystrophy. Neuromuscul Disord 26:73–84
Yoon SH, Sugamori KS, Grynpas MD, Mitchell J (2018) Effect of 25-hydroxyvitamin D deficiency and its interaction with prednisone treatment on musculoskeletal health in growing Mdx mice. Calcif Tissue Int. https://doi.org/10.1007/s00223-018-0423-3
Dempster DW, Compston JE, Drezner MK et al (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28:2–17
Schakman O, Kalista S, Barbé C et al (2013) Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 45:2163–2172
Acharyya S, Villalta SA, Bakkar N et al (2007) Interplay of IKK/NF-κB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Investig 117:889–901
Chazaud B, Sonnet C, Lafuste P et al (2003) Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163:1133–1143
Radley HG, Grounds MD (2006) Cromolyn administration (to block mast cell degranulation) reduces necrosis of dystrophic muscle in mdx mice. Neurobiol Dis 23:387–397
Hodgetts S, Radley H, Davies M, Grounds MD (2006) Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFb1; function with Etanercept in mdx mice. Neuromuscul Disord 16:591–602
Chiu HC, Chiu CY, Yang RS et al. (2018) Preventing muscle wasting by osteoporosis drug alendronate in vitro and in myopathy models via sirtuin-3 down-regulation. J Cachexia Sarcopenia Muscle. https://doi.org/10.1002/jcsm.12289 (Epub ahead of print)
Gordon BS, Delgado Diaz DC, Kostek MC (2013) Resveratrol decreases inflammation and increases utrophin expression in the mdx mouse model of Duchenne muscular dystrophy. Clin Nutr 32:104–111
Chalkiadaki A, Igarashi M, Nasamu AS et al (2014) Muscle-specific SIRT1 gain-of-function increases slow-twitch fibers and ameliorates pathophysiology in a mouse model of Duchenne muscular dystrophy. PLoS Genet 10:e1004490
Gafni RI, McCarthy EF, Hatcher T et al (2002) Recovery from osteoporosis through skeletal growth: early bone mass acquisition has little effect on adult bone density. FASEB J 16:736–738
Leonard MB (2007) Glucocorticoid-induced osteoporosis in children: impact of the underlying disease. Pediatrics 119(Supplement 2):S166–S174
Salle BL, Rauch F, Travers R et al (2002) Human fetal bone development: histomorphometric evaluation of the proximal femoral metaphysis. Bone 30:823–828
Zeitlin L, Rauch F, Travers R,et al (2006) The effect of cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta type V. Bone 38:13–20
Bauss F, Wagner M, Hothorn LH (2002) Total administered dose of ibandronate determines its effects on bone mass and architecture in ovariectomized aged rats. J Rheumatol 29:990–998
Ersek A, Santo AIE, Vattakuzhi Y et al (2016) Strain dependent differences in glucocorticoid-induced bone loss between C57BL/6J and CD-1 mice. Sci Rep 6:36513
Bouvard B, Gallois Y, Legrand E et al (2013) Glucocorticoids reduce alveolar and trabecular bone in mice. Joint Bone Spine 80:77–81
Postnov A, De Schutter T, Sijbers J et al (2009) Glucocorticoid-induced osteoporosis in growing mice is not prevented by simultaneous intermittent PTH treatment. Calcif Tissue Int 85:530–537
He M, Wang J, Wang G et al (2016) Effect of glucocorticoids on osteoclast function in a mouse model of bone necrosis. Mol Med Rep 14:1054–1060
Lin S, Huang J, Zheng L et al (2014) Glucocorticoid-induced osteoporosis in growing rats. Calcif Tissue Int 95:362–373
Misof BM, Roschger P, McMillan HJ et al (2016) Histomorphometry and bone matrix mineralization before and after bisphosphonate treatment in boys with Duchenne muscular dystrophy: a paired transiliac biopsy study. J Bone Miner Res 31:1060–1069
Rauch F, Travers R, Munns C, Glorieux FH (2004) Sclerotic metaphyseal lines in a child treated with pamidronate: histomorphometric analysis. J Bone Miner Res 19:1191–1193
Acknowledgements
This work was supported by a grant to MDG and JM from the Canadian Institutes of Health Research (CIHR MOP-123265), JC received support from a Canada Graduate Scholarship-Master’s from CIHR, S-HY received support from the Ontario Student Opportunity Trust Funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Jinghan Chen, Sung-Hee Yoon, Marc D. Grynpas, Jane Mitchell declare they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All animal care procedures were reviewed and approved by the Animal Care Committee of the University of Toronto. Human informed consent statements are not applicable since this is an animal study.
Rights and permissions
About this article
Cite this article
Chen, J., Yoon, SH., Grynpas, M.D. et al. Pre-treatment with Pamidronate Improves Bone Mechanical Properties in Mdx Mice Treated with Glucocorticoids. Calcif Tissue Int 104, 182–192 (2019). https://doi.org/10.1007/s00223-018-0482-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0482-5