Abstract
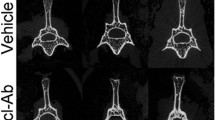
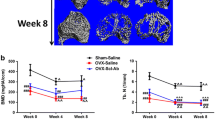
Open fractures remain a challenge in orthopedics. Current strategies to intervene are often inadequate, particularly in severe fractures or when treatment is delayed. Sclerostin is a negative regulator of bone growth and sclerostin-neutralizing antibodies (Scl-Ab) can increase bone mass and strength. The application of these antibodies to improve orthopedic repair has shown varied results, and may be dependent on the location and severity of the bony injury. We examined Scl-Ab treatment within an established rat osteotomy model with periosteal stripping analogous to open fracture repair. In one study, Scl-Ab was given 25 mg/kg bi-weekly, either from the time of fracture or from 3 weeks post-fracture up to an end-point of 12 weeks. A second study treated only delayed union open fractures that did not show radiographic union by week 6 post-fracture. Outcome measures included radiographic union, microCT analysis of bone volume and architecture, and histology. In the first study, Scl-Ab given from either 0 or 3 weeks significantly improved callus bone volume (+52%, p < 0.05 and +58%, p < 0.01) at 12 weeks, as well as strength (+48%, p < 0.05 and +70%, p < 0.05). Despite these improvements, union rate was not changed. In the second study treating only established delayed fractures, bony callus volume was similarly increased by Scl-Ab treatment; however, this did not translate to increased biomechanical strength or union improvement. Sclerostin antibody treatment has limited effects on the healing of challenging open fractures with periosteal stripping, but shows the greatest benefits on callus size and strength with earlier intervention.







Similar content being viewed by others
References
Semenov M, Tamai K, He X (2005) SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem 280:26770–26775
Poole KE, van Bezooijen RL, Loveridge N et al (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
van Bezooijen RL, Roelen BA, Visser A et al (2004) Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199:805–814
Li X, Ominsky MS, Warmington KS et al (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24:578–588
Li X, Warmington KS, Niu QT et al (2010) Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res 25:2371–2380
Ominsky MS, Li C, Li X et al (2011) Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res 26:1012–1021
Ominsky MS, Vlasseros F, Jolette J et al (2010) Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25:948–959
McColm J, Hu L, Womack T et al (2014) Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res 29:935–943
McClung MR, Grauer A, Boonen S et al (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370:412–420
Padhi D, Jang G, Stouch B et al (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26:19–26
Jawad MU, Fritton KE, Ma T et al (2013) Effects of sclerostin antibody on healing of a non-critical size femoral bone defect. J Orthop Res 31:155–163
Virk MS, Alaee F, Tang H et al (2013) Systemic administration of sclerostin antibody enhances bone repair in a critical-sized femoral defect in a rat model. J Bone Joint Surg Am 95:694–701
Alaee F, Virk MS, Tang H et al (2014) Evaluation of the effects of systemic treatment with a sclerostin neutralizing antibody on bone repair in a rat femoral defect model. J Orthop Res 32:197–203
Agholme F, Li X, Isaksson H et al (2010) Sclerostin antibody treatment enhances metaphyseal bone healing in rats. J Bone Miner Res 25:2412–2418
McDonald MM, Morse A, Mikulec K et al (2012) Inhibition of sclerostin by systemic treatment with sclerostin antibody enhances healing of proximal tibial defects in ovariectomized rats. J Orthop Res 30:1541–1548
McGee-Lawrence ME, Ryan ZC, Carpio LR et al (2013) Sclerostin deficient mice rapidly heal bone defects by activating beta-catenin and increasing intramembranous ossification. Biochem Biophys Res Commun 441:886–890
Cui L, Cheng H, Song C et al (2013) Time-dependent effects of sclerostin antibody on a mouse fracture healing model. J Musculoskelet Neuronal Interact 13:178–184
Li C, Ominsky MS, Tan HL et al (2011) Increased callus mass and enhanced strength during fracture healing in mice lacking the sclerostin gene. Bone 49:1178–1185
Morse A, Yu NY, Peacock L et al (2015) Endochondral fracture healing with external fixation in the Sost knockout mouse results in earlier fibrocartilage callus removal and increased bone volume fraction and strength. Bone 71:155–163
Suen PK, He YX, Chow DH et al (2014) Sclerostin monoclonal antibody enhanced bone fracture healing in an open osteotomy model in rats. J Orthop Res 32:997–1005
Feng G, Chang-Qing Z, Yi-Min C et al (2015) Systemic administration of sclerostin monoclonal antibody accelerates fracture healing in the femoral osteotomy model of young rats. Int Immunopharmacol 24:7–13
Tagil M, McDonald MM, Morse A et al (2010) Intermittent PTH(1–34) does not increase union rates in open rat femoral fractures and exhibits attenuated anabolic effects compared to closed fractures. Bone 46:852–859
Schindeler A, Yu NY, Cheng TL et al (2015) Local delivery of the cationic steroid antibiotic CSA-90 enables osseous union in a rat open fracture model of Staphylococcus aureus infection. J Bone Joint Surg Am 97:302–309
Caetano-Lopes J, Lopes A, Rodrigues A et al (2011) Upregulation of inflammatory genes and downregulation of sclerostin gene expression are key elements in the early phase of fragility fracture healing. PLoS ONE 6:e16947
Dean DB, Watson JT, Jin W et al (2010) Distinct functionalities of bone morphogenetic protein antagonists during fracture healing in mice. J Anat 216:625–630
Cheng TL, Schindeler A, Little DG (2016) BMP-2 delivered via sucrose acetate isobutyrate (SAIB) improves bone repair in a rat open fracture model. J Orthop Res 34:1168–1176
Acknowledgements
The authors received materials support (Scl-AbIII) for this study from Amgen Inc. and UCB Pharma (Brussels, Belgium).
Author contributions
Study design: DGL, MMM, ML, HZK. Study conduct: AM, MMM, LP, KM. Data collection: AM, LP, KM, TLC. Data interpretation: AM, MMM, DGL, TLC. Drafting manuscript: AM and AS. Revising manuscript content: AM, AS, DGL, MMM, TLC, HZK, ML. Approving final version of manuscript: all authors. AM takes responsibility for the integrity of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors received materials support (Scl-AbIII) for this study from Amgen Inc. and UCB (Brussels, Belgium). Prof Little has received additional funding and materials support from Amgen Inc. and UCB Pharma for pre-clinical research separate to this submission. Prof Little has received funding support from Novartis Pharma AG, N8 Medical and Celgene for pre-clinical research separate to this submission. Dr McDonald received salary support from the IBMS Greg Mundy Fellowship and receives a Practitioner Fellowship (National Health & Medical Research Council). Authors Dr Liu and Prof Ke are or have been employees of Amgen Inc. Prof Ke is an employee of UCB, Alyson Morse, Aaron Schindler, Lauren Peacock, Kathy Mikulec, and Tegan L Cheng have no conflict of interest.
Human and Animal Rights and Informed Consent
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Morse, A., McDonald, M.M., Schindeler, A. et al. Sclerostin Antibody Increases Callus Size and Strength but does not Improve Fracture Union in a Challenged Open Rat Fracture Model. Calcif Tissue Int 101, 217–228 (2017). https://doi.org/10.1007/s00223-017-0275-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0275-2