Abstract
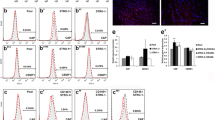
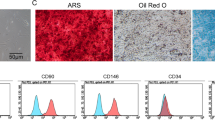
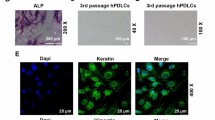
It has been previously reported that caveolin-1 (Cav-1) knockout mice exhibit increased bone size and stiffness. However, the expression and role of Cav-1 on periodontal tissue is poorly understood. The aim of this study was to investigate the immunohistochemical expression of Cav-1 in the mouse periodontium and explore the role of Cav-1 on osteoblastic and cementoblastic differentiation in human periodontal ligament cells (hPDLCs), cementoblasts, and osteoblasts. To reveal the molecular mechanisms of Cav-1 activity, associated signaling pathways were also examined. Immunolocalization of Cav-1 was studied in mice periodontal tissue. Differentiation was evaluated by ALP activity, alizarin red S staining, and RT-PCR for marker genes. Signal transduction was analyzed using Western blotting and confocal microscopy. Cav-1 expression was observed in hPDLCs, cementoblasts, and osteoblasts of the periodontium both in vivo and in vitro. Inhibition of Cav-1 expression by methyl-β-cyclodextrin (MβCD) and knockdown of Cav-1 by siRNA promoted osteoblastic and cementoblastic differentiation by increasing ALP activity, calcium nodule formation, and mRNA expression of differentiation markers in hPDLCs, cementoblasts, and osteoblasts. Osteogenic medium-induced BMP-2 and BMP-7 expression, and phosphorylation of Smad1/5/8 were enhanced by MβCD and siRNA knockdown of Cav-1, which was reversed by BMP inhibitor noggin. MβCD and Cav-1 siRNA knockdown increased OM-induced AMPK, Akt, GSK3β, and CREB phosphorylation, which were reversed by Ara-A, a specific AMPK inhibitor. Moreover, OM-induced activation of p38, ERK, JNK, and NF-κB was enhanced by Cav-1 inhibition. This study demonstrates, for the first time, that Cav-1 is expressed in developing periodontal tissue and in vitro in periodontal-related cells. Cav-1 inhibition positively regulates osteoblastic differentiation in hPDLCs, cementoblasts, and osteoblasts via BMP, AMPK, MAPK, and NF-κB pathway. Thus, Cav-1 inhibition may be a novel molecular target for therapeutic approaches in periodontitis or osteolytic disease.








Similar content being viewed by others
References
Choi MH, Noh WC, Park JW, Lee JM, Suh JY (2011) Gene expression pattern during osteogenic differentiation of human periodontal ligament cells in vitro. J Periodontal Implant Sci 41:167–175
Mamalis A, Markopoulou C, Lagou A, Vrotsos I (2011) Oestrogen regulates proliferation, osteoblastic differentiation, collagen synthesis and periostin gene expression in human periodontal ligament cells through oestrogen receptor beta. Arch Oral Biol 56:446–455
Fujii S, Maeda H, Wada N, Kano Y, Akamine A (2006) Establishing and characterizing human periodontal ligament fibroblasts immortalized by SV40T-antigen and hTERT gene transfer. Cell Tissue Res 324:117–125
Xu WP, Shiba H, Mizuno N, Uchida Y, Mouri Y, Kawaguchi H, Kurihara H (2004) Effect of bone morphogenetic proteins-4, -5 and -6 on DNA synthesis and expression of bone-related proteins in cultured human periodontal ligament cells. Cell Biol Int 28:675–682
Hidaka T, Nagasawa T, Shirai K, Kado T, Furuichi Y (2012) FGF-2 induces proliferation of human periodontal ligament cells and maintains differentiation potentials of STRO-1(+)/CD146(+) periodontal ligament cells. Arch Oral Biol 57:830–840
Yu Y, Mu J, Fan Z, Lei G, Yan M, Wang S, Tang C, Wang Z, Yu J, Zhang G (2012) Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem Cell Biol 137:513–525
Lee JH, Um S, Jang JH, Seo BM (2012) Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res 348:475–484
Chung JH, Kim YS, Noh K, Lee YM, Chang SW, Kim EC (2014) Deferoxamine promotes osteoblastic differentiation in human periodontal ligament cells via the nuclear factor erythroid 2-related factor-mediated antioxidant signaling pathway. J Periodontal Res 49:563–573
Lee SK, Choi HI, Yang YS, Jeong GS, Hwang JH, Lee SI, Kang KH, Cho JH, Chae JM, Lee SK, Kim YC, Kim EC (2009) Nitric oxide modulates osteoblastic differentiation with heme oxygenase-1 via the mitogen activated protein kinase and nuclear factor-kappaB pathways in human periodontal ligament cells. Biol Pharm Bull 32:1328–1334
Kook YA, Lee SK, Son DH, Kim Y, Kang KH, Cho JH, Kim SC, Kim YS, Lee HJ, Lee SK, Kim EC (2009) Effects of substance P on osteoblastic differentiation and heme oxygenase-1 in human periodontal ligament cells. Cell Biol Int 33:424–428
Cho JH, Lee SK, Lee JW, Kim EC (2010) The role of heme oxygenase-1 in mechanical stress-and lipopolysaccharide-induced osteogenic differentiation in human periodontal ligament cells. Angle Orthod 80:552–559
Lee YM, Shin SI, Shin KS, Lee YR, Park BH, Kim EC (2011) The role of sirtuin 1 in osteoblastic differentiation in human periodontal ligament cells. J Periodontal 46:712–721
Lofthouse RA, Davis JR, Frondoza CG, Jinnah RH, Hungerford DS, Hare JM (2001) Identification of caveolae and detection of caveolin in normal human osteoblasts. J Bone Joint Surg 83:124–129
Wang Y, Maciejewski BS, Drouillard D, Santos M, Hokenson MA, Hawwa RL, Huang Z, Sanchez-Esteban J (2010) A role for caveolin-1 in mechanotransduction of fetal type II epithelial cells. J Physiol Lung Cell Mol Physiol 298:L775–L783
Yamaguchi T, Naruishi K, Arai H, Nishimura F, Takashiba S (2008) IL-6/sIL-6R enhances cathepsin B and L production via caveolin-1-mediated JNK-AP-1 pathway in human gingival fibroblasts. J Cell Physiol 217:423–432
Solomon KR, Adolphson LD, Wank DA, McHugh KP, Hauschka PV (2000) Caveolae in human and murine osteoblasts. J Bone Miner Res 15:2391–2401
Solomon KR, Danciu TE, Adolphson LD, Hecht LE, Hauschka PV (2000) Caveolin-enriched membrane signaling complexes in human and murine osteoblasts. J Bone Miner Res 15:2380–2390
Nohe A, Keating E, Underhill TM, Knaus P, Petersen NO (2005) Dynamics and interaction of caveolin-1 isoforms with BMP-receptors. J Cell Sci 118:643–650
Sawada N, Taketani Y, Amizuka N, Ichikawa M, Ogawa C, Nomoto K, Nashiki K, Sato T, Arai H, Isshiki M, Segawa H, Yamamoto H, Miyamoto K, Takeda E (2007) Caveolin-1 in extracellular matrix vesicles secreted from osteoblasts. Bone 41:52–58
Sotgia F, Williams TM, Cohen AW, Minetti C, Pestell RG, Lisanti MP (2005) Caveolin-1-deficient mice have an increased mammary stem cell population with upregulation of Wnt/beta-catenin signaling. Cell Cycle 4:1808–1816
Rubin J, Schwartz Z, Boyan BD, Fan X, Case N, Sen B, Drab M, Smith D, Aleman M, Wong KL, Yao H, Jo H, Gross TS (2007) Caveolin-1 knockout mice have increased bone size and stiffness. J Bone Miner Res 22:1408–1418
Jung SY, Kwak JO, Kim HW, Kim DS, Ryu SD, Ko CB, Cha SH (2005) Calcium sensing receptor forms complex with and is up-regulated by caveolin-1 in cultured human osteosarcoma (Saos-2) cells. Exp Mol Med 37:91–100
Kitagawa M, Kudo Y, Iizuka S, Ogawa I, Abiko Y, Miyauchi M, Takata T (2006) Effect of F-spondin on cementoblastic differentiation of human periodontal ligament cells. Biochem Biophys Res Commun 349:1050–1056
Hayami T, Zhang Q, Kapila Y, Kapila S (2007) Dexamethasone’s enhancement of osteoblastic markers in human periodontal ligament cells is associated with inhibition of collagenase expression. Bone 40:93–104
Afzal F, Polak J, Buttery L (2004) Endothelial nitric oxide synthase in the control of osteoblastic mineralizing activity and bone integrity. J Pathol 202:503–510
Brun-Heath I, Ermonval M, Chabrol E, Xiao J, Palkovits M, Lyck R, Miller F, Couraud PO, Mornet E, Fonta C (2011) Differential expression of the bone and the liver tissue non-specific alkaline phosphatase isoforms in brain tissues. Cell Tissue Res 343:521–536
Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T (2009) Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3-E1 cells through endothelial NOS and BMP-2 expression. Am J Physiol Endocrinol Metab 296:E139–E146
Saygin NE, Giannobile WV, Somerman MJ (2000) Molecular and cell biology of cementum. Periodontol 2000(24):73–98
Sodek J (2000) McKee MD (2000) Molecular and cellular biology of alveolar bone. Periodontol 24:99–126
Iizuka N, Suzuki A, Nozawa-Inoue K, Kawano Y, Nandasena BG, Okiji T, Maeda T (2009) Differential cell-specific location of Cav-1 and Ca(2+)-ATPase in terminal Schwann cells and mechanoreceptive Ruffini endings in the periodontal ligament of the rat incisor. J Anat 214:267–274
Murshed M, Schinke T, McKee MD, Karsenty G (2009) Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol 165:625–630
Narisawa S, Fröhlander N, Millán JL (1997) Inactivation of two mouse alkaline phosphatase genes and establishment of a model of infantile hypophosphatasia. Dev Dyn 208:432–446
Baker N, Zhang G, You Y, Tuan RS (2012) Caveolin-1 regulates proliferation and osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem 113:3773–3787
Rey-Barroso J, Alvarez-Barrientos A, Rico-Leo E, Contador-Troca M, Carvajal-Gonzalez JM, Echarri A, Del Pozo MA, Fernandez-Salguero PM (2014) The Dioxin receptor modulates Caveolin-1 mobilization during directional migration: role of cholesterol. Cell Commun Signal 12:57–76
Liang L, Yu JF, Wang Y, Wang G, Ding Y (2008) Effect of estrogen receptor beta on the osteoblastic differentiation function of human periodontal ligament cells. Arch Oral Biol 53:553–557
Diercke K, König A, Kohl A, Lux CJ, Erber R (2012) Human primary cementoblasts respond to combined IL-1β stimulation and compression with an impaired BSP and CEMP-1 expression. Eur J Cell Biol 91:402–412
Cormier C (1995) Markers of bone metabolism. Curr Opin Rheumatol 7:243–248
Takizawa N, Sawada S, Chosa N, Ishisaki A, Naruishi K (2013) Secreted caveolin-1 enhances periodontal inflammation by targeting gingival fibroblasts. Biomed Res 34:1–11
Anitha M, Shahnavaz N, Qayed E, Joseph I, Gossrau G, Mwangi S, Sitaraman SV, Greene JG, Srinivasan S (2010) BMP2 promotes differentiation of nitrergic and catecholaminergic enteric neurons through a Smad1-dependent pathway. Am J Physiol Gastrointest Liver Physiol 298:G375–G383
Morsczeck C (2006) Gene expression of runx2, Osterix, c-fos, DLX-3, DLX-5, and MSX-2 in dental follicle cells during osteogenic differentiation in vitro. Calcif Tissue Int 78:98–102
Moon RT, Bowerman B, Boutros M, Perrimon N (2002) The promise and perils of wnt signaling through beta-catenin. Science 296:1644–1646
Sotgia F, Williams TM, Cohen AW, Minetti C, Pestell RG, Lisanti MP (2005) Caveolin-1-deficient mice have an increased mammary stem cell population with upregulation of Wnt/beta-catenin signaling. Cell Cycle 4(12):1808–1816
Inanc B, Elcin AE, Elcin YM (2006) Osteogenic induction of human periodontal ligament fibroblasts under two- and three-dimensional culture conditions. Tissue Eng 12:257–266
Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T (2007) Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3–E1 cells. BMC Cell Biol 8:51–62
Horike N, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Kamata H, Nishiyama K, Uchijima Y, Kurihara Y, Kurihara H, Asano T (2008) AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J Biol Chem 283:33902–33910
Hada N, Okayasu M, Ito J, Nakayachi M, Hayashida C, Kaneda T, Uchida N, Muramatsu T, Koike C, Masuhara M, Sato T, Hakeda Y (2012) Receptor activator of NF-κB ligand-dependent expression of caveolin-1 in osteoclast precursors, and high dependency of osteoclastogenesis on exogenous lipoprotein. Bone 50:226–236
Park JH, Han HJ (2009) Caveolin-1 plays important role in EGF-induced migration and proliferation of mouse embryonic stem cells: involvement of PI3 K/Akt and ERK. Am J Physiol Cell Physiol 297:C935–C944
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MEST) (No. 2012R1A5A2051384), and a grant from the Korean Health Technology R&D Project through the KHIDI, funded by the Ministry of Health & Welfare, Republic of Korea (HI14C0175).
Author Contributions
Conceived and designed the experiments: SYL, ESC, and ECK. Performed the experiments: SYL, HBY, KSL, and CHB. Analyzed the data: SYL, JJ, KSL, and JKY. Contributed reagents/materials/analysis tools: JJ. Wrote the paper: SYL and JKY. Revised the manuscript: ECK and HJC. Read and approved the final manuscript: SYL, JKY, JJ, ESC, and ECK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing financial interests.
Human and Animal Rights and Informed Consent
This study was ethically approved by the Institutional Animal Care and Use Committee of Kyung Hee University (Seoul, Korea) and performed in accordance with the criteria defined by the rules of the committee.
Additional information
Lee SY and Yi JK have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lee, SY., Yi, JK., Yun, HM. et al. Expression of Caveolin-1 in Periodontal Tissue and Its Role in Osteoblastic and Cementoblastic Differentiation In Vitro. Calcif Tissue Int 98, 497–510 (2016). https://doi.org/10.1007/s00223-015-0095-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0095-1