Abstract
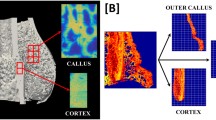
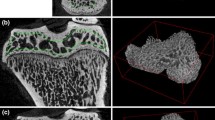
About 5–10 % of all bone fractures suffer from delayed healing, which may lead to non-union. Bone morphogenetic proteins (BMPs) can be used to induce differentiation of osteoblasts and enhance the formation of the bony callus, and bisphosphonates help to retain the newly formed callus. The aim of this study was to investigate if scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) can identify differences in the mineral composition of the newly formed bone compared to cortical bone from a non-fractured control. Moreover, we investigate whether the use of BMPs and bisphosphonates—alone or combined—may have an effect on bone mineralization and composition. Twelve male Sprague–Dawley rats at 9 weeks of age were randomly divided into four groups and treated with (A) saline, (B) BMP-7, (C) bisphosphonates (Zoledronate), and (D) BMP-7 + Zoledronate. The rats were sacrificed after 6 weeks. All samples were imaged using SEM and chemically analyzed with EDS to quantify the amount of C, N, Ca, P, O, Na, and Mg. The Ca/P ratio was the primary outcome. In the fractured samples, two areas of interest were chosen for chemical analysis with EDS: the callus and the cortical bone. In the non-fractured samples, only the cortex was analyzed. Our results showed that the element composition varied to a small extent between the callus and the cortical bone in the fractured bones. However, the Ca/P ratio did not differ significantly, suggesting that the mineralization at all sites is similar 6 weeks post-fracture in this rat model.




Similar content being viewed by others
References
Skinner HCW (2005) Mineralogy of bone. In: Selinus O, Alloway B, Centeno JA, Finkelman RB, Fuge R, Lindh U, Smedley P (eds) Essentials of medical geology: impacts of the natural environment on public health. Elsevier, Boston, pp 667–693
Chen PY, Toroian D, Price PA, McKittrick J (2011) Minerals form a continuum phase in mature cancellous bone. Calcif Tissue Int 88:351–361
Murugan R, Ramakrishna S, Panduranga Rao K (2006) Nanoporous hydroxy-carbonate apatite scaffold made of natural bone. Mater Lett 60:2844–2847
Allen MR, Burr DB (2007) Mineralization, microdamage, and matrix: how bisphosphonates influence material properties of bone. BoneKEy Osteovision 4:49–60
Turunen MJ, Saarakkala S, Rieppo L, Helminen HJ, Jurvelin JS, Isaksson H (2011) Comparison between infrared and Raman spectroscopic analysis of maturing rabbit cortical bone. Appl Spectrosc 65:595–603
Victoria G, Petrisor B, Drew B, Dick D (2009) Bone stimulation for fracture healing: what’s all the fuss? Indian J Orthop 43:117–120
Groeneveld E, Burger E (2000) Bone morphogenetic proteins in human bone regeneration. Eur J Endocrinol 142:9–21
Bosemark P, Isaksson H, McDonald MM, Little DG, Tägil M (2013) Augmentation of autologous bone graft by a combination of bone morphogenic protein and bisphosphonate increased both callus volume and strength. Acta Orthop 84:106–111
Jeppsson C, Åstrand J, Tägil M, Aspenberg P (2003) A combination of bisphosphonate and BMP additives in impacted bone allografts. Acta Orthop 74:483–489
Rodan GA (2000) Therapeutic approaches to bone diseases. Science 289:1508–1514
Li EC, Davis LE (2003) Zoledronic acid: a new parenteral bisphosphonate. Clin Ther 25:2669–2708
Burr DB, Miller L, Grynpas M, Li J, Boyde A, Mashiba T, Hirano T, Johnston CC (2003) Tissue mineralization is increased following 1-year treatment with high doses of bisphosphonates in dogs. Bone 33:960–969
Shapiro R, Heaney RP (2003) Co-dependence of calcium and phosphorus for growth and bone development under conditions of varying deficiency. Bone 32:532–540
Tzaphlidou M (2008) Bone architecture: collagen structure and calcium/phosphorus maps. J Biol Phys 34:39–49
Kourkoumelis N, Balatsoukas I, Tzaphlidou M (2012) Ca/P concentration ratio at different sites of normal and osteoporotic rabbit bones evaluated by Auger and energy dispersive X-ray spectroscopy. J Biol Phys 38:279–291
Tzaphlidou M, Speller R, Royle G, Griffiths J (2006) Preliminary estimates of the calcium/phosphorus ratio at different cortical bone sites using synchrotron microCT. Phys Med Biol 51:1849–1855
Midura RJ, Vasanji A, Su X, Wang A, Midura SB, Gorski JP (2007) Calcospherulites isolated from the mineralization front of bone induce the mineralization of type I collagen. Bone 41:1005–1016
Zimmermann KA, LeBlanc JM, Sheets KT, Fox RW, Gatenholm P (2011) Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater Sci Eng C 31:43–49
Tagil M, McDonald MM, Morse A, Peacock L, Mikulec K, Amanat N, Godfrey C, Little DG (2010) Intermittent PTH(1-34) does not increase union rates in open rat femoral fractures and exhibits attenuated anabolic effects compared to closed fractures. Bone 46:852–859
Grynpas MD, Pritzker KP, Hancock RG (1987) Neutron activation analysis of bulk and selected trace elements in bones using a low flux SLOWPOKE reactor. Biol Trace Elem Res 13:333–344
Åkesson K, Grynpas MD, Hancock RGV, Odselius R, Obrant KJ (1994) Energy-dispersive X-ray microanalysis of the bone mineral content in human trabecular bone: a comparison with ICPES and neutron activation analysis. Calcif Tissue Int 55:236–239
Yu S, Yu Z, Wang G, Han J, Ma X, Dargusch MS (2011) Biocompatibility and osteoconduction of active porous calcium-phosphate films on a novel Ti-3Zr-2Sn-3Mo-25Nb biomedical alloy. Colloids Surf B 85:103–115
Little DG, McDonald M, Bransford R, Godfrey CB, Amanat N (2005) Manipulation of the anabolic and catabolic responses with OP-1 and zoledronic acid in a rat critical defect model. J Bone Miner Res 20:2044–2052
Gross KA, Berndt CC (2002) Biomedical application of apatites. Rev Mineral Geochem 48:631–672
Ren FZ, Leng Y (2012) Carbonated apatite, type-A or type-B? Key Eng Mater 493:293–297
Wopenka B, Pasteris JD (2005) A mineralogical perspective on the apatite in bone. Mater Sci Eng C 25:131–143
Pasteris JD, Wopenka B, Freeman JJ, Rogers K, Valsami-Jones E, van der Houwen JAM, Silva MJ (2004) Lack of OH in nanocrystalline apatite as a function of degree of atomic order: implications for bone and biomaterials. Biomaterials 25:229–238
Cohen AM, Talmi YP, Floru S, Tsigelman R, Kalmanovitz M, Zohar Y, Djaldetti M (1991) X-ray microanalysis of ossified auricles in Addison’s disease. Calcif Tissue Int 48:88–92
Green LJ, Eick JD, Miller WA, Leitner JW (1970) Electron microprobe analysis of Ca, P, and Mg in mandibular bone. J Dent Res 49:608–615
Gentleman E, Swain RJ, Evans ND, Boonrungsiman S, Jell G, Ball MD, Shean TA, Oyen ML, Porter A, Stevens MM (2009) Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nat Mater 8:763–770
Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G (1998) Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone 23:187–196
Henricson A, Hulth A, Johnell O (1987) The cartilaginous fracture callus in rats. Acta Orthop Scand 58:244–248
Preininger B, Checa S, Molnar FL, Fratzl P, Duda GN, Raum K (2011) Spatial-temporal mapping of bone structural and elastic properties in a sheep model following osteotomy. Ultrasound Med Biol 37:474–483
Markel MD, Wikenheiser MA, Chao EY (1990) A study of fracture callus material properties: relationship to the torsional strength of bone. J Orthop Res 8:843–850
Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Pleshko Camacho N, Bostrom MP (2007) Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 41:928–936
Acknowledgments
The Project was funded from the European Commission (FRACQUAL-293434), the Swedish Agency for Innovation Systems, Vinnova, Carl Trygger foundation (CTS 12:200, CTS 13:185), and the foundations of Greta and Johan Kock. The authors would like to thank Prof. Reine Wallenberg and Ms. Gunnel Karlsson (Centre for Analysis and Synthesis, LU) for the technical support and scientific discussions. The authors would also like to thank Ms. Mea Pelkonen, MSc (Department of Orthopaedics, Lund University) for her valuable assistance with the sample preparation.
Conflict of interest
Christina Perdikouri, Magnus Tägil, and Hanna Isaksson state that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
The study was approved by the local animal ethics committee at Lund University (ethical permit number: M216-08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perdikouri, C., Tägil, M. & Isaksson, H. Characterizing the Composition of Bone Formed During Fracture Healing Using Scanning Electron Microscopy Techniques. Calcif Tissue Int 96, 11–17 (2015). https://doi.org/10.1007/s00223-014-9930-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-014-9930-z