Abstract
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation tool with potential for managing neuromuscular fatigue, possibly due to alterations in corticospinal excitability. However, inconsistencies in intra- and inter- individual variability responsiveness to tDCS limit its clinical use. Emerging evidence suggests harnessing homeostatic metaplasticity induced via tDCS may reduce variability and boost its outcomes, yet little is known regarding its influence on neuromuscular fatigue in healthy adults. We explored whether cathodal tDCS (ctDCS) prior to exercise combined with anodal tDCS (atDCS) could augment corticospinal excitability and attenuate neuromuscular fatigue. 15 young healthy adults (6 males, 22 ± 4 years) participated in four pseudo-randomised neuromodulation sessions: sham stimulation prior and during exercise, sham stimulation prior and atDCS during exercise, ctDCS prior and atDCS during exercise, ctDCS prior and sham stimulation during exercise. The exercise constituted an intermittent maximal voluntary contraction (MVC) of the right first dorsal interosseous (FDI) for 10 min. Neuromuscular fatigue was quantified as an attenuation in MVC force, while motor evoked potential (MEP) amplitude provided an assessment of corticospinal excitability. MEP amplitude increased during the fatiguing exercise, whilst across time, force decreased. There were no differences in MEP amplitudes or force between neuromodulation sessions. These outcomes highlight the ambiguity of harnessing metaplasticity to ameliorate neuromuscular fatigue in young healthy individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromuscular fatigue is characterised by an exercise-induced decline in maximal voluntary muscle force (Taylor et al. 2016). It is a prevalent incapacitating symptom recognised as causing decrements in quality of life and disability (Kluger et al. 2013). Both the peripheral and central nervous systems of the body are established to contribute to neuromuscular fatigue (Wan et al. 2017). During neuromuscular fatigue, a gradual decrease in maximal voluntary activation of muscle due to a decline in neural drive occurs centrally (Gandevia 2001). Whilst peripherally, alterations distal or within the neuromuscular junction are apparent (Wan et al. 2017). Prior research has also revealed alterations in the excitability of the corticospinal tract during neuromuscular fatigue (Taylor et al. 1996). In fact, corticospinal excitability is enhanced during single-joint fatiguing exercise, possibly reflecting an increase in motor output to the muscle to counteract the progressive difficulty in maintaining contractile force (Gandevia et al. 1996).
The use of non-invasive brain stimulation techniques such as transcranial direct current stimulation (tDCS) can modify corticospinal excitability (Nitsche and Paulus 2000). tDCS is a portable, non-invasive, painless method of neuromodulation that when employed, delivers continual, low direct electrical current through electrodes on the scalp (Nitsche and Paulus 2000). The effects of tDCS are predominantly a consequence of polarity-specific bidirectional alteration of resting membrane potential (Liebetanz et al. 2002). Anodal tDCS (atDCS) increases cerebral excitability by eliciting subthreshold membrane depolarisation (Nitsche and Paulus 2000). On the other hand, cathodal tDCS (ctDCS) causes a decrease in cerebral excitability and membrane hyperpolarisation (Nitsche and Paulus 2000). Prolonged use of atDCS and ctDCS induces lasting effects similar to persistent forms of activity-dependent synaptic plasticity mediated through strong activation of N-methyl-D-aspartate-type glutamate receptors, namely, long-term potentiation (LTP) and long-term depression (LTD) respectively (Bliss and Lomo 1973; Lüscher and Malenka 2012).
Metaplasticity is referred to regulation of neural activity by which induction of synaptic change is dependent on previous synaptic activity (Abraham and Bear 1996). It operates to preserve synaptic activity within a dynamic range to aid in the integration of temporally dispersed episodes of synaptic change (Abraham and Bear 1996). The Bienenstock-Cooper-Munro theory presents a theoretical explanation on metaplasticity, implying that the threshold for synaptic modification dynamically and bi-directionally varies as a function of prior activity (Bienenstock et al. 1982). Previous LTP activity shifts the modification threshold so that further LTP induction is more difficult, and subsequently increases the likelihood of LTD (Abraham and Tate 1997). The opposite is noticed with previous LTD activity (Abraham and Tate 1997). Prior studies have used two distinct blocks of tDCS over the motor cortex to harness metaplasticity and improve skill acquisition and motor learning in a healthy population (Christova et al. 2015; Fujiyama et al. 2017). For instance, Christova and colleagues found ctDCS preceding atDCS applied concurrently during a grooved pegboard test reduced the completion time of the task when compared to sham condition (Christova et al. 2015). Ultimately, employing priming ctDCS to reduce the modification threshold appears to enhance both corticospinal excitability and functional outcomes of succeeding atDCS (Christova et al. 2015; Fujiyama et al. 2017). Presently, there is a paucity of studies exploring the interaction between metaplastic neuromodulation and neuromuscular fatigue using tDCS.
Currently, no universally efficacious therapy exists for offsetting neuromuscular fatigue (Wan et al. 2017). Given the partial efficacy of existing treatments and the degree to which neuromuscular fatigue can impact an individual’s activities of daily living, exploring the relationship between metaplastic neuromodulation and neuromuscular fatigue may be important for uncovering a non-pharmacological and non-invasive treatment approach. Specifically, we explored priming ctDCS prior to single-joint fatiguing exercise combined with atDCS on corticospinal excitability and neuromuscular fatigue in young, healthy adults. We hypothesised that ctDCS primed atDCS applied concurrently with fatiguing exercise would augment corticospinal excitability facilitation and attenuate neuromuscular fatigue compared to atDCS primed by sham stimulation (stDCS).
Experimental procedures
Participants
Fifteen young healthy volunteers were recruited for the study (6 males, mean age ± SD, 22 ± 4 years). The sample size estimation was performed using G*Power V.3.1.9.4. Due to the design of the study (i.e., repeated measures), it was determined that for an effect size of 0.4 (medium), alpha error of 0.05, power of 0.8, using 95% confidence interval and an expected correlation of the repeated measures of 0.7, 15 participants were required for the study (Cohen 1988). According to the Edinburgh handedness questionnaire, all but one participant were right-handed (handedness Laterality Index, 0.84 ± 0.3), with one individual being ambidextrous (Oldfield 1971). All participants used their right hand during the study sessions. Before participation, volunteers were screened for contraindications to transcranial magnetic stimulation (TMS) (e.g., metallic implants in the skull, cardiac pacemaker, neurological condition, substance abuse, history of seizures, epilepsy, and/or pregnancy). Participants were instructed to refrain from consuming caffeine 4 h prior to the experimental session since ingestion of caffeine has been reported to affect fatigability and performance outcomes (Doherty et al. 2004). The protocol was conducted in accordance with the Declaration of Helsinki and was approved by the Human Research Ethics Committee at the University of Adelaide. The protocol was not registered in a database. Written informed consent was obtained from all participants before involvement. Participants were reimbursed for their time upon completion of the study.
Experimental set-up
Participants sat with their right elbow fixed approximately 90°, pronated forearm aligned on a horizontal surface, and index finger positioned adjacent a force transducer (MLP 100; Transducer Techniques, Temecula, CA). A custom manipulandum was used to restrain the forearm and wrist. Responses evoked from the right first dorsal interosseous (FDI) muscle were recorded via two electromyography (EMG) surface electrodes (Ag/AgCl) arranged over the muscle in a belly-tendon montage. An additional two grounding electrodes were placed on the wrist and forearm to minimise electrical noise. EMG was amplified (1000x) and band-pass filtered (20 Hz – 1 kHz) (1902; Cambridge Electronic Design [CED], UK) prior to digitisation via a 1401 interface at 2 kHz and stored offline.
Experimental protocol
Participants attended the laboratory for four experimental sessions, each separated by at least 7 days to avoid any long-term effects of tDCS (Peters et al. 2013; Reis et al. 2009). To control for diurnal influence, experimental sessions were completed in the afternoon between the hours of 12 pm and 6 pm (Ridding and Ziemann 2010). During each session, the tDCS paradigm was changed and the order was counterbalanced. The order of these sessions was pseudorandomised and double-blinded with the aid of a separate experimenter, meaning both the participant and main experimenter were blinded to tDCS polarities. Within each of the four experimental sessions, tDCS was applied twice. The initial tDCS functioned to prime subsequent tDCS which was applied during exercise. Priming neuromodulation was either stDCS or ctDCS, whilst stDCS or atDCS was applied during the exercise. Hence, the four experimental sessions were: (1) stDCS-stDCS, (2) stDCS-atDCS, (3) ctDCS-atDCS, (4) ctDCS-stDCS. The stDCS-stDCS and ctDCS-stDCS sessions were control conditions, providing evidence on whether the effects of ctDCS-atDCS were explicitly related to the metaplastic neuromodulation intervention, or merely a result of the fatiguing exercise task.
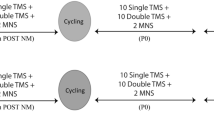
Before employing tDCS, baseline measures of force and corticospinal excitability were taken (Fig. 1(A). Maximal voluntary contraction (MVC) force was established by calculating the average force of three brief (∼5-second) maximal FDI abduction contractions (no more than 5% variation in force between contractions), each separated by 30-seconds. Baseline corticospinal excitability was measured via eliciting 15 single TMS pulses and 3 peripheral nerve stimulations (PNS). TMS were elicited prior to PNS. Participants then received priming tDCS at rest; ctDCS or stDCS. At 2 min and 8 min post-priming, measurements (15 TMS and 3 PNS) were taken to assess the influence of priming on corticospinal excitability. 10 min succeeding the termination of priming, test tDCS stimulation begun; atDCS or stDCS (Fig. 1(B). An inter-stimulation interval of 10 min deemed suitable to ensure test tDCS was executed throughout the after-effects of priming tDCS (Monte-Silva et al. 2010). At the start, middle and end of all tDCS periods, participants reported sensations from the tDCS electrodes. 30-seconds following test tDCS commencement, corticospinal excitability measurements (15 TMS and 3 PNS) were repeated. Participants then completed the fatiguing exercise comprising 10 intermittent 30-second MVCs. Between each set (30-seconds), 5 TMS and 1 PNS were elicited. Participants were verbally instructed to start and stop contracting as well as verbally encouraged to perform maximally during the exercise. Force and EMG output were presented on a computer monitor for visual feedback. Post-exercise measurements (15 TMS, 3 PNS followed by 2 brief MVCs separated by 30-seconds) were completed immediately following exercise and repeated 10 min and 20 min after the exercise concluded to gauge neuromuscular fatigue recovery and tDCS after-effects (Fig. 1(C).
Experimental protocol schematic. (A) Baseline and priming tDCS (B) Fatiguing exercise and test tDCS (C) Post-exercise/recovery. tDCS: transcranial direct current stimulation. ctDCS: cathodal transcranial direct current stimulation. stDCS: sham transcranial direct current stimulation. atDCS: anodal transcranial direct current stimulation. Sec: second. Mins: minutes. TMS: transcranial magnetic stimulation. PNS: peripheral nerve stimulation. 5s: five second. 30s: 30 second. MVC: maximal voluntary contraction
tDCS
Constant direct current was delivered by a pair of 35 cm2 saline-soaked, synthetic sponge electrodes connected to a battery-powered direct current stimulator (NeuroConn DC Stimulator Plus, Germany). A bipolar tDCS electrode montage appears to optimally enhance motor cortex excitability (Nitsche and Paulus 2000) and hence, was used in the current study. The active electrode was centred on the left primary motor cortex (M1), specifically over the representational field of the right FDI muscle. The reference electrode was positioned over the contralateral supraorbital region. tDCS polarity refers to the electrode over the left M1. The M1 hotspot for tDCS was determined as the area of the left hemisphere exhibiting the largest motor evoked potential (MEP) amplitude at 40–60% of maximum TMS stimulator output. The current of the tDCS was set at an intensity of 1 mA (Batsikadze et al. 2013; Jamil et al. 2017; Xian et al. 2023). To replicate previous work, priming ctDCS was applied for 15 min, whilst atDCS during exercise (test tDCS) was administered for 12 min (Fig. 1) (Xian et al. 2023). For stDCS, although the electrode montage was identical to that of genuine tDCS administration, current was only delivered for 30-seconds. Thus, no corticospinal excitability changes were induced during stDCS (Kristiansen et al. 2021). In both real and stDCS, the current was ramped-up and down over 8-seconds at the start and end of the stimulation to inhibit electrical transients.
TMS
TMS was applied to the left M1 to evaluate the excitability of projections from the cortical representation of the right FDI muscle. Single-pulse TMS stimuli were delivered using a standard figure-of-8 magnetic coil (9 cm external wing diameter) connected to a Magstim BiStim unit (MagStim Company, Dyfed, UK). The coil was positioned tangentially over the brain at a 45° angle to the sagittal plane to deliver a posterior-anterior current flow, and to optimally elicit MEPs in the FDI. The amplitude of MEP was used to assess corticospinal excitability. All TMS pulses were delivered while participants contracted their right FDI muscle at 5% of their maximum EMG (as determined during baseline MVC trials). An active state was chosen for practicality since a lower stimulation intensity is required to consistently produce a MEP if muscle is active (Ngomo et al. 2012). Additionally, and perhaps more importantly, active muscle more closely represents what occurs during a task (i.e., fatiguing exercise). The FDI motor hotspot was established by mapping (at 40–60% of maximum stimulator output) for the area that resembled the largest MEP amplitude. The position of the coil was marked on the tDCS electrodes and scalp with permanent marker to aid in consistent coil positioning. These markings were continuously monitored throughout the protocol. Active motor threshold (AMT) was defined as the lowest TMS stimulus intensity necessary to elicit a MEP discernible from background EMG signal in 3 out of 5 trials (Sidhu et al. 2017). TMS intensity was set at 120% of AMT for all measurement blocks (stDCS-stDCS: 56 ± 9%; stDCS-atDCS: 55 ± 11%; ctDCS-atDCS: 55 ± 10%; ctDCS-stDCS: 54 ± 8%; of maximum stimulator output, One-way ANOVA main effect of session: P = 0.89) to ensure effective activation of the FDI during the experiment.
PNS
The right ulnar nerve was peripherally stimulated via a bipolar bar electrode probe connected to a constant-current stimulator (DS7A; Digitimer, Hertfordshire, UK). Upon establishing the optimal position, the probe was secured so that the cathode was angled distally. The optimal position was established as the site eliciting the greatest compound muscle action potential (M-wave) in resting FDI at 10 mA current. To ascertain the maximum M-wave (Mmax), stimulation intensity was incrementally increased by 5 mA until the M-wave amplitude ceased to rise. Stimuli were delivered at a test intensity of 120% of the intensity necessary to produce Mmax (stDCS-stDCS: 27 ± 6 mA; stDCS-atDCS: 26 ± 6 mA; ctDCS-atDCS: 28 ± 7 mA; ctDCS-stDCS: 28 ± 6 mA; One-way ANOVA main effect of session: P = 0.50).
Data analysis
MVC data were manually analysed using offline recordings on Spike2 software (Version 6.18; CED, UK). Force amplitude was measured during brief (∼5-second) MVCs at baseline and post-exercise and averaged across trials at each time point. Mean force (from initial peak to just prior to the end of contraction) was measured for each of the 30-second MVCs during the exercise. The root mean squared EMG was measured for every 5% maximum EMG contraction to ensure all measurements were taken at a constant EMG level.
Peak-to-Peak MEP and Mmax amplitudes were calculated in millivolts using offline recordings on Spike2 software. MEP amplitudes were measured between 20 and 50 milliseconds after the TMS pulse, whilst Mmax amplitudes were measured between 3 and 30 milliseconds following PNS. MEP amplitude at individual time points were determined as the mean amplitude across all trials in the measurement block. MEPs were normalised to Mmax (MEP % Mmax) to study corticospinal changes whilst excluding changes at the level of the muscle.
Statistical analysis
IBM SPSS Statistics software (Version 24; Chicago, USA) was used for the statistical analyses. Separate linear mixed model (LMM) analyses with factors time and neuromodulation (stDCS-stDCS vs. stDCS-atDCS vs. ctDCS-atDCS vs. ctDCS-stDCS) were used to assess main effects and interactions. For MEP, LMM analyses were performed for baseline and post-priming, 30-seconds post-tDCS and all the fatiguing contraction time points, and baseline and post-exercise. Furthermore, two LMM analyses were completed for force: one for all the fatiguing contraction time points and another for baseline and post-exercise. For all comparisons, normality of the data was validated using Shapiro-Wilk tests. Post hoc tests with Bonferroni’s correction for multiple comparisons were employed to probe significant main and interaction effects. One-way analyses of variance were used for evaluating differences between neuromodulation conditions in time of day, lab temperature and humidity. All data in text and tables are conveyed as mean ± SD. Data in figures are illustrated as box-and-whisker plots, with the “box” depicting the median and the 25th and 75th quartiles, and the “whisker” highlighting the 5th and 95th percentile. The value of alpha was set at ≤ 0.05.
Results
Participant characteristics are displayed in Table 1. No adverse reactions to tDCS were reported by participants. Participants were unable to distinguish stDCS from real tDCS since sensations related with real stimulation were also communicated during stDCS. The sensations reported comprised needling, warmth, burning, stinging, prickling, itchiness, and tingling. Most described sensations at the beginning of tDCS but, felt nothing when asked in the middle and at the end of the stimulation (see Supplementary Material 1). There were no differences between neuromodulation conditions in time of day (1:26 pm ± 1.2 h; P \(\ge\) 0.679), lab temperature (21.6 ± 1.1 °C; P \(\ge\) 0.426), or humidity (40.0 ± 5.1%; P \(\ge\) 0.124). All data were normally distributed with equal variances assumed.
Corticospinal excitability
Post-priming, there were no main effects of time (F2,10 = 0.017, P = 0.984), neuromodulation (F3,13 = 2.00, P = 0.163) nor interaction between time and neuromodulation (F6,87 = 1.12, P = 0.360) on MEP amplitudes (Fig. 2(A)). During exercise (Fig. 2(B)) however, there was a main effect of time (F9,123 = 4.82, P < 0.001) on MEP amplitudes but, not neuromodulation (F3,55 = 1.27, P = 0.293) nor interaction between time and neuromodulation (F27,380 = 1.02, P = 0.444). MEPs were facilitated compared to 30-seconds post-test tDCS across all neuromodulation conditions during fatiguing contraction two through to the ninth contraction (P ≤ 0.022). Similarly, there was a main effect of time (F3,33 = 4.14, P = 0.013) on MEP amplitudes post-exercise (Fig. 2(C)) but, not neuromodulation (F3,48 = 0.396, P = 0.756) nor interaction between time and neuromodulation (F9,131 = 1.67, P = 0.103). Across all neuromodulation conditions, MEP increased from baseline immediately post-exercise (0 min) (P = 0.008) but returned to baseline 10 min post-exercise (P = 0.923).
Post-priming, there were no main effects of time (F2,27 = 0.532, P = 0.593), neuromodulation (F3,55 = 1.77 P = 0.163) nor any interaction between time and neuromodulation (F6,85 = 0.688, P = 0.660) on Mmax (baseline Mmax across all neuromodulation conditions, 15.7 ± 0.58 mV). However, during exercise, there was a main effect of time (F9,126 = 25.4, P < 0.001) on Mmax but, not neuromodulation (F3,56 = 1.13, P = 0.343). There was also an interaction between time and neuromodulation (F27,378 = 1.88, P = 0.006) on Mmax during exercise. Mmax attenuated from 30-seconds post-test tDCS across all neuromodulation conditions (P ≤ 0.004) during fatiguing contraction one through to the ninth contraction. Additionally, at 30-seconds post-test tDCS, Mmax was greater in stDCS-atDCS (16.6 ± 4.0 mV, P = 0.034) and ctDCS-stDCS (16.6 ± 4.0 mV, P = 0.036) neuromodulation conditions when compared to stDCS-stDCS (13.3 ± 4.0 mV). Post-exercise, there was a main effect of time (F3,39 = 21.9, P < 0.001) on Mmax but not neuromodulation (F3,53 = 1.40, P = 0.252) nor interaction between time and neuromodulation (F9,127 = 0.91, P = 0.519). Across all neuromodulation conditions, Mmax attenuated from baseline immediately post-exercise (0 min) (P < 0.05) but returned to baseline from 10 min post-exercise (P ≥ 0.218).
There were no main effects of time (F2,27 = 0.745, P = 0.484), neuromodulation (F3,55 = 1.01 P = 0.395), nor any interaction between time and neuromodulation (F6,84 = 1.74, P = 0.121) on background EMG post-priming. Similarly, there were no main effects of time (F9,504 = 0.761, P = 0.652), neuromodulation (F3,56 = 1.01 P = 0.397), nor any interaction between time and neuromodulation (F27,504 = 1.20, P = 0.225) on background EMG during exercise. There were also no main effects of time (F3,42 = 0.305, P = 0.821), neuromodulation (F3,56 = 0.783 P = 0.509), nor any interaction between time and neuromodulation (F9,127 = 0.971, P = 0.467) on background EMG post-exercise. Hence, all TMS and PNS measurements were essentially taken when participants consistently held a 5% maximum EMG contraction.
Mean MEP amplitude (expressed as percentage of Mmax) post-priming (A), during fatiguing exercise (B), and post-exercise (C) from fifteen young healthy adults displayed across neuromodulation conditions (stDCS-stDCS, stDCS-atDCS, ctDCS-atDCS, ctDCS-stDCS) over time. Bonferroni’s post hoc tests: * indicates significant difference from 30s post-test tDCS (P < 0.05); # denotes significant difference from baseline (P < 0.05). Data are represented as box-and-whisker plots, with the “box” depicting the median and the 25th and 75th quartiles, and the “whisker” highlighting the 5th and 95th percentile. tDCS: transcranial direct current stimulation. stDCS: sham tDCS. atDCS: anodal tDCS. ctDCS: cathodal tDCS. stDCS-stDCS: stDCS applied during priming and exercise. stDCS-atDCS: stDCS applied during priming and atDCS applied during exercise. ctDCS-atDCS: ctDCS applied during priming and atDCS applied during exercise. ctDCS-stDCS: ctDCS applied during priming and stDCS applied during exercise. MEP: motor evoked potential. Mmax: maximal muscle compound action potential. MEP(%Mmax): MEP normalised to Mmax. Min: minute. S: seconds
Neuromuscular fatigue
While there was an expected main effect of time (F9,122 = 18.7, P < 0.001), there was no main effect of neuromodulation (F3,53 = 0.067, P = 0.977) nor interaction between time and neuromodulation (F27,379 = 0.484, P = 0.987) on MVC force during fatiguing exercise (Fig. 3(A)). Across all neuromodulation conditions, when compared to the beginning of the exercise (Fatigue 1), MVC force progressively declined from the third through to the tenth contraction (P < 0.05) (Fig. 3(A)). Post-exercise (Fig. 3(B)), there was a main effect of time (F3,33 = 58.7, P < 0.001) on MVC force but, not neuromodulation (F3,48 = 0.141, P = 0.935). There was also an interaction between time and neuromodulation (F9,129 = 2.11, P = 0.033) on MVC force post-exercise. Force remained attenuated at 0-, 10- and 20-minutes post-exercise across all neuromodulation conditions (P < 0.001) (Fig. 3(B)). There was no statistically significant difference between the two brief MVCs performed immediately following the completion of the fatiguing exercise (0-minutes post-exercise) (P = 0.4).
Mean MVC force during- (A) and post- (B) fatiguing exercise displayed over time and across neuromodulation conditions (stDCS-stDCS, stDCS-atDCS, ctDCS-atDCS, ctDCS-stDCS) for fifteen young healthy adults. Bonferroni’s post hoc tests: * indicates significant difference from fatiguing contraction 1 (Fatigue 1) (P < 0.05); # denotes significant difference from baseline (P < 0.05). Data are represented as box-and-whisker plots, with the “box” depicting the median and the 25th and 75th quartiles, and the “whisker” highlighting the 5th and 95th percentile. tDCS: transcranial direct current stimulation. stDCS: sham tDCS. atDCS: anodal tDCS. ctDCS: cathodal tDCS. stDCS-stDCS: stDCS applied during priming and exercise. stDCS-atDCS: stDCS applied during priming and atDCS applied during exercise. ctDCS-atDCS: ctDCS applied during priming and atDCS applied during exercise. ctDCS-stDCS: ctDCS applied during priming and stDCS applied during exercise. N: newton. Min: minute
Discussion
Main findings
The current study is the first to explore the interaction between metaplastic neuromodulation and neuromuscular fatigue in young, healthy individuals. Data on tDCS-induced modulation of MEP amplitudes in young healthy participants showed no significant shift in corticospinal excitability with ctDCS-atDCS compared to stDCS-atDCS, ctDCS-stDCS or excitability facilitation induced by the fatiguing exercise itself (stDCS-stDCS). Likewise, there was no benefit of tDCS, either primed or not primed, for the development of neuromuscular fatigue during exercise. Since this study revealed no significant benefit of ctDCS-atDCS on enhancing corticospinal excitability or reducing neuromuscular fatigue during exercise, the prospect of harnessing metaplasticity to ameliorate neuromuscular fatigue in young healthy individuals remains elusive.
tDCS effects on corticospinal excitability
Our current understanding of an enhancement of MEP amplitudes during fatiguing maximal efforts (i.e. MVCs) is attributed to increases in corticospinal excitability (Taylor et al. 1996), arising as a counteractive mechanism to boost contractile force as neuromuscular fatigue develops (Gandevia et al. 1996). The notion that corticospinal excitability increases during single-joint fatiguing exercise (Benwell et al. 2007; Otieno et al. 2019) is verified in the present study by an enhancement of MEP amplitudes in the stDCS-stDCS condition. The Bienenstock-Cooper-Munro theory states that the threshold for synaptic modification dynamically and bi-directionally adjusts as a function of former activity (Bienenstock et al. 1982). Although it is understood that the induction of excitability modification is sensitive to the state of the network enforced by prior synaptic activity (Abraham and Bear 1996; Abraham and Tate 1997; Bienenstock et al. 1982; Siebner et al. 2004; Lang et al. 2004), we found no differences in MEP amplitudes between the explored neuromodulation conditions. Hence, our hypothesis of an augmentation in corticospinal excitability facilitation with ctDCS primed atDCS applied simultaneously with fatiguing exercise is not confirmed in this study.
Maximal compound muscle action potential, or Mmax, signifies full activation of the motor neurone pool of a muscle (Magladery et al. 1951). Studies reveal a decrease in Mmax amplitude in the presence of muscular fatigue (Crone et al. 1999). In the present study, it is likely that to some degree, the attenuation in Mmax is a result of the exercise protocol inducing fatigue. At a physiological level, an impairment of neuromuscular propagation is a probable cause for this attenuated Mmax (Fuglevand et al. 1993). However, there were several instances across several participants (6 data points out of a total of 600 in the data set: 1% of data) whereby the MEP was greater than Mmax. Since it is not physiologically possible for TMS to markedly recruit more motoneurons than supramaximal stimulation of motoneuron axons, we suspect that there were methodological issues in obtaining Mmax (e.g. movement of the stimulation probe from the optimal position on the nerve) during the fatiguing exercise task. In any case, analysis of MEP (unnormalised) resulted in similar outcomes compared to MEP (normalised to Mmax) – suggesting that the MEP outcome as reported in the current study stands independent of muscle changes. Interestingly, in comparison to stDCS-stDCS neuromodulation, Mmax at the beginning of the fatiguing exercise (30-seconds post-test tDCS) was greater in stDCS-atDCS and ctDCS-stDCS conditions. The underlying reason for the differences in Mmax between sessions is not clear and may require further investigation in future work.
Notably, there was a lack of difference in corticospinal excitability between stDCS-atDCS and stDCS-stDCS neuromodulation in this study that conflicts with previous investigations exploring physiological and behavioural outcomes of tDCS (Cogiamanian et al. 2007; Kidgell et al. 2013; Nitsche and Paulus 2000, 2001; Nitsche et al. 2002; Williams et al. 2013). Earlier studies examining the application of atDCS during muscle activity have revealed increases in corticospinal excitability (Hendy and Kidgell 2013; Kim and Ko 2013). The lack of difference between stDCS-atDCS and stDCS-stDCS in the current study compared to similar research in the field (Cogiamanian et al. 2007; Williams et al. 2013) may be due to variation in methodologies and study designs. For example, unlike the present study, many tDCS investigations have failed to blind the experimenter involved in the research (Cappa 2008; Marangolo et al. 2011; Monti et al. 2008; Norise et al. 2017; Shah-Basak et al. 2015); increasing the risk of bias. Additionally, the experimental parameters of tDCS vary among studies. Factors such as timing, intensity, and duration of tDCS application are known to influence tDCS outcomes (Sellaro et al. 2016). In this study, tDCS was applied at an intensity of 1 mA yet, other studies in the area commonly employ a tDCS intensity of 1.5–2 mA (Abdelmoula et al. 2019; Cogiamanian et al. 2007; Fujiyama et al. 2017; Kan et al. 2013). It has been established however, that higher tDCS intensities does not necessarily increase corticospinal excitability if more than 1.5 mA is administered (Kan et al. 2013). Likewise, the duration of tDCS application in this study was 15 min of priming and 12 min during the exercise task but, studies reporting an effect of stDCS-atDCS apply tDCS during performance of a motor task for 20 min (Christova et al. 2015; Fujiyama et al. 2017). Batsikadze and colleagues have demonstrated that an increase in tDCS intensity or duration is not strictly associated with an enhancement of its efficacy but may in fact alter the direction of its effects (Batsikadze et al. 2013). Inter-individual variability of response to tDCS may also contribute to the conflicting outcomes of the present study to that of prior investigations (Chew et al. 2015; Xian et al. 2023). In fact, it has been observed that the after-effects of neuromodulation are variable within and between participants in relation to direction, duration, and magnitude (Huang et al. 2017) and influenced by electrode and skull characteristics (Antonenko et al. 2021). A variety of factors may play a role in the neuromodulation response variability including sex, attention, physical activity levels and optimal stimulation dose (Fertonani and Miniussi 2017; Guerra et al. 2020; Huang et al. 2017; Li et al. 2015; López-Alonso et al. 2014; Ridding and Ziemann 2010; Rudroff et al. 2020). Hence, future studies exploring the relationship between corticospinal excitability and different tDCS parameters are necessary to decipher the most appropriate arrangement for enhancing tDCS outcomes (Amann et al. 2022).
tDCS effects on neuromuscular fatigue
The ability of neuromodulation to mutually modify motor performance and corticospinal excitability highlights the possibility of exploiting neuromodulation to influence mechanisms of neuromuscular fatigue (Christova et al. 2015; Fujiyama et al. 2017). The present study reveals an attenuation of MVC force during exercise regardless of the neuromodulation condition. MVC remained significantly lower than baseline across all four conditions at 20 min post-exercise. Kan et al. (2013) reported similar results in their study, wherein atDCS had no effect on MVC strength or time-to-task-failure (TTF) of the elbow flexors (Kan et al. 2013). However, unlike the delivery of tDCS during exercise in the present study, Kan et al. (2013) applied atDCS prior to exercise. Conversely, Williams et al. (2013) reached differing conclusions in their study, whereby application of atDCS during fatiguing exercise led to an improvement in performance. A fundamental distinction in the protocol that could justify the contradictory result concerns the quantification of neuromuscular fatigue. Williams et al. (2013) assessed neuromuscular fatigue by TTF in submaximal isometric contractions (as opposed to intermittent MVCs in the current study). Consequently, it is probable that amelioration of neuromuscular fatigue via tDCS is task dependent and regulated, at least partially, by exercise intensity.
Since development of neuromuscular fatigue is related to an increase in corticospinal excitability (Benwell et al. 2007; Otieno et al. 2019), a parallel between fatigability and excitability of outcomes is expected. Earlier research has demonstrated an attenuation of neuromuscular fatigue upon delivery of atDCS concurrently with exercise (Oki et al. 2016; Williams et al. 2013). Yet, investigations on priming ctDCS prior to concurrent application of atDCS during a task have illustrated enhanced corticospinal excitability and motor performance when compared to atDCS with no priming (stDCS) (Christova et al. 2015; Fujiyama et al. 2017). Hence, we anticipated that excitability modulation via priming ctDCS prior to atDCS delivered simultaneously during fatiguing exercise would attenuate neuromuscular fatigue compared to atDCS primed by stDCS. However, onset of neuromuscular fatigue and lack of recovery in force post-exercise was consistent across all investigated tDCS paradigms as no differential tDCS modulation of corticospinal excitability was present in this study.
Limitations
The current study has some considerations that should be addressed. Firstly, it could be disputed that capitalisation of metaplasticity was not effective as the priming alone caused no modification in MEP. Yet, even when priming stimulation fails to produce noticeable variations in excitability, subsequent synaptic alterations have been shown to occur (Abraham 2008; Abraham and Bear 1996; Sidhu 2021). Moreover, several variables are well-established in influencing the outcomes of tDCS. Although we are aware of hormones and the potent regulatory role they play in plasticity (Abraham 2008; Ansdell et al. 2019), we did not control for the menstrual cycle in female participants and is considered a limitation. Additionally, sex differences in fatigability are documented (Ansdell et al. 2020; Hunter 2014). Females are commonly described as having greater resistance to neuromuscular fatigue because of greater availability of oxygen during exercise (Ansdell et al. 2019, 2020). Regarding study techniques, neuronavigation was not employed in our study. Neuronavigation systems allow for accurate identification of the brain area of interest by utilisation of an individual’s magnetic resonance imaging (MRI) data (Sparing et al. 2010). With the absence of MRI, neuronavigation systems may be paired with anatomical data and optically tracked frameless stereotaxic systems to allow for continual tracking of a hotspot (Orringer et al. 2012). While neuronavigation is common in TMS studies for precise positioning of the magnetic coil, it is of greater importance in studies exploring brain regions other than M1 (e.g., frontal cortex) (Herwig et al. 2001; Sparing et al. 2010). A further limitation of the present work is the fact that the effectiveness of neuromodulation (i.e., tDCS) and neuromuscular fatigue appear to be muscle and task dependent (Otieno et al. 2022; Simione et al. 2018). Hence, the outcomes of the current study in the FDI may not necessarily be extrapolated to muscle groups that are commonly used during activities of daily living and with a different topographical representation in M1. It could also be argued that the relatively homogenous young healthy study sample is a limitation. However, investigating the basic neurophysiological mechanisms of neuromuscular fatigue in a healthy cohort has broader significance for the field. Lastly, there is some indication of increased variability if fewer than 20 simultaneous MEP responses are averaged (Biabani et al. 2018). Thus, the 5 TMS-evoked MEP responses during fatiguing exercise, and 15 at every other time point, should be acknowledged.
Conclusion
This work offers a novel application of tDCS to modulate corticospinal excitability and fatigability in young, healthy adults. In contrast to prior investigations, we observed no differential modulation of corticospinal excitability or neuromuscular fatigue with delivery of atDCS during isometric single-joint fatiguing exercise. Consequently, priming ctDCS prior to fatiguing exercise combined with atDCS failed to augment corticospinal excitability facilitation or attenuate neuromuscular fatigue. Overall, the present results highlight some limitations in the use of tDCS to attenuate muscle fatigability. Further exploration to establish the efficacy of tDCS and the underlying neurophysiological mechanisms in mitigating neuromuscular fatigue in both normal and pathological settings is warranted.
Data availability
Data will be made available on reasonable request.
Abbreviations
- AMT:
-
Active motor threshold
- atDCS:
-
Anodal transcranial direct current stimulation
- ctDCS:
-
Cathodal transcranial direct current stimulation
- EMG:
-
Electromyography
- FDI:
-
First dorsal interosseous
- LMM:
-
Linear mixed model
- LTD:
-
Long term depression
- LTP:
-
Long term potentiation
- M1:
-
Primary motor cortex
- MEP:
-
Motor evoked potential
- Mmax:
-
Maximum M-wave
- MVC:
-
Maximal voluntary contraction
- PNS:
-
Peripheral nerve stimulation
- stDCS:
-
Sham transcranial direct current stimulation
- tDCS:
-
Transcranial direct current stimulation
- TMS:
-
Transcranial magnetic stimulation
- TTF:
-
Time-to-task-failure
Abbreviations
- Corticospinal Excitability:
-
The excitability of the pathway from the cortical site of neuronal depolarization to spinal motoneuron depolarization
- Long Term Depression (LTD):
-
A phenomenon where the cumulative activation of inputs to specific neural pathways produces a decrease in the excitability of these neurons
- Long Term Potentiation (LTP):
-
A phenomenon where repeated electrical stimulation of inputs to specific neural pathways produces an increase in the excitability of these neurons
- Motor Evoked Potential (MEP):
-
The electrical signals recorded from the descending motor pathways or from muscles following stimulation of motor pathways within the brain
- Neuromodulation:
-
The alteration of neuronal and synaptic properties by neurons themselves, substances released by neurons, or by technology that acts directly upon nerves
- Neuromuscular Fatigue:
-
Multifactorial phenomenon that can occur in various sites along the112 pathway of force production, causing a reduced ability to generate a desired muscle force
- Neuroplasticity:
-
Also known as neural plasticity or brain plasticity, is a process that involves adaptive structural and functional changes to the brain
- Trancranial Direct Current Stimulation (tDCS):
-
A non-invasive form of neuromodulation used to modulate corticospinal excitability by use of constant, low, direct current delivered via electrodes on the head
- Transcranial magnetic stimulation (TMS):
-
A non-invasive form of brain stimulation used to test and modify corticospinal excitability via electromagnetic induction
References
Abdelmoula A, Baudry S, Duchateau J (2019) Anodal transcranial direct current stimulation does not influence the neural adjustments associated with fatiguing contractions in a hand muscle. Eur J Appl Physiol 119:597–609
Abraham WC (2008) Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci 9:387
Abraham WC, Bear MF (1996) Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19:126–130
Abraham WC, Tate WP (1997) Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol 52:303–323
Amann M, Sidhu SK, McNeil CJ, Gandevia SC (2022) Critical considerations of the contribution of the corticomotoneuronal pathway to central fatigue. J Physiol 600:5203–5214
Ansdell P, Brownstein CG, Škarabot J, Hicks KM, Simoes DCM, Thomas K, Howatson G, Hunter SK et al (2019) Menstrual cycle-associated modulations in neuromuscular function and fatigability of the knee extensors in eumenorrheic women. J Appl Physiol (1985) 126:1701–1712
Ansdell P, Škarabot J, Atkinson E, Corden S, Tygart A, Hicks KM, Thomas K, Hunter SK et al (2020) Sex differences in fatigability following exercise normalised to the power-duration relationship. J Physiol 598:5717–5737
Antonenko D, Grittner U, Saturnino G, Nierhaus T, Thielscher A, Flöel A (2021) Inter-individual and age-dependent variability in simulated electric fields induced by conventional transcranial electrical stimulation. NeuroImage 224:117413. https://doi.org/10.1016/j.neuroimage.2020.117413Epub 2020 Oct 1. PMID: 33011418
Baecke JA, Burema J, Frijters JE (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36:936–942
Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA (2013) Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 591:1987–2000
Benwell NM, Mastaglia FL, Thickbroom GW (2007) Differential changes in long-interval intracortical inhibition and silent period duration during fatiguing hand exercise. Exp Brain Res 179:255–262
Biabani M, Farrell M, Zoghi M, Egan G, Jaberzadeh S (2018) The minimal number of TMS trials required for the reliable assessment of corticospinal excitability, short interval intracortical inhibition, and intracortical facilitation. Neurosci Lett 674:94–100
Bienenstock EL, Cooper LN, Munro PW (1982) Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci 2:32–48
Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356
Cappa SF (2008) Current to the brain improves word-finding difficulties in aphasic patients. J Neurol Neurosurg Psychiatry 79:364
Chew T, Ho KA, Loo CK (2015) Inter- and intra-individual variability in response to Transcranial Direct Current Stimulation (tDCS) at varying current intensities. Brain Stimul 8:1130–1137
Christova M, Rafolt D, Gallasch E (2015) Cumulative effects of anodal and priming cathodal tDCS on pegboard test performance and motor cortical excitability. Behav Brain Res 287:27–33
Cogiamanian F, Marceglia S, Ardolino G, Barbieri S, Priori A (2007) Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur J Neurosci 26:242–249
Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Routledge. https://doi.org/10.4324/9780203771587
Crone C, Johnsen LL, Hultborn H, Orsnes GB (1999) Amplitude of the maximum motor response (Mmax) in human muscles typically decreases during the course of an experiment. Exp Brain Res 124:265–270
Doherty M, Smith P, Hughes M, Davison R (2004) Caffeine lowers perceptual response and increases power output during high-intensity cycling. J Sports Sci 22:637–643
Fertonani A, Miniussi C (2017) Transcranial electrical stimulation: what we know and do not know about mechanisms. Neuroscientist 23:109–123
Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM (1993) Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol 460:549–572
Fujiyama H, Hinder MR, Barzideh A, Van de Vijver C, Badache AC, Manrique CM, Reissig P, Zhang X et al (2017) Preconditioning tDCS facilitates subsequent tDCS effect on skill acquisition in older adults. Neurobiol Aging 51:31–42
Gandevia SC (2001) Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789
Gandevia SC, Allen GM, Butler JE, Taylor JL (1996) Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol 490(Pt 2):529–536
Guerra A, López-Alonso V, Cheeran B, Suppa A (2020) Variability in non-invasive brain stimulation studies: reasons and results. Neurosci Lett 719:133330
Hendy AM, Kidgell DJ (2013) Anodal tDCS applied during strength training enhances motor cortical plasticity. Med Sci Sports Exerc 45:1721–1729
Herwig U, Padberg F, Unger J, Spitzer M, Schönfeldt-Lecuona C (2001) Transcranial magnetic stimulation in therapy studies: examination of the reliability of standard coil positioning by neuronavigation. Biol Psychiatry 50:58–61
Huang YZ, Lu MK, Antal A, Classen J, Nitsche M, Ziemann U, Ridding M, Hamada M et al (2017) Plasticity induced by non-invasive transcranial brain stimulation: a position paper. Clin Neurophysiol 128:2318–2329
Hunter SK (2014) Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol (Oxf) 210:768–789
Jamil A, Batsikadze G, Kuo HI, Labruna L, Hasan A, Paulus W, Nitsche MA (2017) Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J Physiol 595:1273–1288
Kan B, Dundas JE, Nosaka K (2013) Effect of transcranial direct current stimulation on elbow flexor maximal voluntary isometric strength and endurance. Appl Physiol Nutr Metab 38:734–739
Kidgell DJ, Daly RM, Young K, Lum J, Tooley G, Jaberzadeh S, Zoghi M, Pearce AJ (2013) Different current intensities of anodal transcranial direct current stimulation do not differentially modulate motor cortex plasticity. Neural Plast 2013, 603502
Kim GW, Ko MH (2013) Facilitation of corticospinal tract excitability by transcranial direct current stimulation combined with voluntary grip exercise. Neurosci Lett 548:181–184
Kluger BM, Krupp LB, Enoka RM (2013) Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology 80:409–416
Kristiansen M, Thomsen MJ, Nørgaard J, Aaes J, Knudsen D, Voigt M (2021) Anodal transcranial direct current stimulation increases corticospinal excitability, while performance is unchanged. PLoS ONE 16:e0254888
Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC (2004) Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry 56:634–639
Li LM, Uehara K, Hanakawa T (2015) The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front Cell Neurosci 9:181
Liebetanz D, Nitsche MA, Tergau F, Paulus W (2002) Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125:2238–2247
López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-Del-Olmo M (2014) Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul 7:372–380
Lüscher C, Malenka RC (2012) NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol 4(6):a005710. https://doi.org/10.1101/cshperspect.a005710
Magladery JW, Porter WE, Park AM, Teasdall RD (1951) Electrophysiological studies of nerve and reflex activity in normal man. IV. The two-neurone reflex and identification of certain action potentials from spinal roots and cord. Bull Johns Hopkins Hosp 88:499–519
Marangolo P, Marinelli CV, Bonifazi S, Fiori V, Ceravolo MG, Provinciali L, Tomaiuolo F (2011) Electrical stimulation over the left inferior frontal gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behav Brain Res 225:498–504
Monte-Silva K, Kuo MF, Liebetanz D, Paulus W, Nitsche MA (2010) Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS). J Neurophysiol 103:1735–1740
Monti A, Cogiamanian F, Marceglia S, Ferrucci R, Mameli F, Mrakic-Sposta S, Vergari M, Zago S et al (2008) Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry 79:451–453
Ngomo S, Leonard G, Moffet H, Mercier C (2012) Comparison of transcranial magnetic stimulation measures obtained at rest and under active conditions and their reliability. J Neurosci Methods 205:65–71
Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527:633–639
Nitsche MA, Paulus W (2001) Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57:1899–1901
Nitsche MA, Liebetanz D, Tergau F, Paulus W (2002) [Modulation of cortical excitability by transcranial direct current stimulation]. Nervenarzt 73:332–335
Norise C, Sacchetti D, Hamilton R (2017) Transcranial Direct Current Stimulation in Post-stroke Chronic Aphasia: the impact of Baseline Severity and Task specificity in a pilot sample. Front Hum Neurosci 11:260
Oki K, Mahato NK, Nakazawa M, Amano S, France CR, Russ DW, Clark BC (2016) Preliminary evidence that Excitatory Transcranial Direct Current Stimulation extends time to Task failure of a sustained, Submaximal muscular contraction in older adults. J Gerontol Biol Sci Med Sci 71:1109–1112
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Orringer DA, Golby A, Jolesz F (2012) Neuronavigation in the surgical management of brain tumors: current and future trends. Expert Rev Med Devices 9(5):491–500
Otieno LA, Opie GM, Semmler JG, Ridding MC, Sidhu SK (2019) Intermittent single-joint fatiguing exercise reduces TMS-EEG measures of cortical inhibition. J Neurophysiol 121:471–479
Otieno LA, Semmler JG, Smith AE, Sidhu SK (2022) Submaximal isometric fatiguing exercise of the elbow flexors has no age-related effect on GABAB-mediated inhibition. J Appl Physiol (1985) 132:167–177
Peters MAK, Thompson B, Merabet LB, Wu AD, Shams L (2013) Anodal tDCS to V1 blocks visual perceptual learning consolidation. Neuropsychologia 51:1234–1239
Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW (2009) Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences 106, 1590
Ridding MC, Ziemann U (2010) Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol 588:2291–2304
Rudroff T, Workman CD, Fietsam AC, Kamholz J (2020) Response variability in Transcranial Direct Current Stimulation: why sex matters. Front Psychiatry 11:585
Sellaro R, Nitsche MA, Colzato LS (2016) The stimulated social brain: effects of transcranial direct current stimulation on social cognition. Ann N Y Acad Sci 1369:218–239
Shah-Basak PP, Norise C, Garcia G, Torres J, Faseyitan O, Hamilton RH (2015) Individualized treatment with transcranial direct current stimulation in patients with chronic non-fluent aphasia due to stroke. Front Hum Neurosci 9:201
Sidhu SK (2021) Remote muscle priming anodal transcranial direct current stimulation attenuates short interval intracortical inhibition and increases time to task failure of a constant workload cycling exercise. Exp Brain Res 239:1975–1985
Sidhu SK, Pourmajidian M, Opie GM, Semmler JG (2017) Increasing motor cortex plasticity with spaced paired associative stimulation at different intervals in older adults. Eur J Neurosci 46:2674–2683
Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC (2004) Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci 24:3379–3385
Simione M, Fregni F, Green JR (2018) The Effect of Transcranial Direct Current Stimulation on Jaw Motor function is Task Dependent: Speech, Syllable Repetition and Chewing. Front Hum Neurosci 12:33. https://doi.org/10.3389/fnhum.2018.00033
Sparing R, Hesse MD, Fink GR (2010) Neuronavigation for transcranial magnetic stimulation (TMS): where we are and where we are going. Cortex 46:118–120
Taylor JL, Butler JE, Allen GM, Gandevia SC (1996) Changes in motor cortical excitability during human muscle fatigue. J Physiol 490(Pt 2):519–528
Taylor JL, Amann M, Duchateau J, Meeusen R, Rice CL (2016) Neural contributions to muscle fatigue: from the brain to the muscle and back again. Med Sci Sports Exerc 48:2294–2306
Wan JJ, Qin Z, Wang PY, Sun Y, Liu X (2017) Muscle fatigue: general understanding and treatment. Exp Mol Med 49:e384
Williams PS, Hoffman RL, Clark BC (2013) Preliminary evidence that anodal transcranial direct current stimulation enhances time to task failure of a sustained submaximal contraction. PLoS ONE 8:e81418
Xian C, Barbi C, Goldsworthy MR, Venturelli M, Sidhu SK (2023) The interaction between metaplastic neuromodulation and fatigue in multiple sclerosis. J Neurol Sci 444:120521
Acknowledgements
This work was supported by the Adelaide Medical School Honours Scholarship awarded to MRB.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Human Research Ethics Committee at the University of Adelaide and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Written informed consent was obtained from all participants before involvement in the study.
Competing interests
The authors declare that they have no conflict of interest. As such, the authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Lindsay Nagamatsu.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boda, M.R., Otieno, L.A., Smith, A.E. et al. Metaplastic neuromodulation via transcranial direct current stimulation has no effect on corticospinal excitability and neuromuscular fatigue. Exp Brain Res 242, 1999–2012 (2024). https://doi.org/10.1007/s00221-024-06874-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-024-06874-z