Abstract
Cognitive flexibility is a core component of executive function and supports the ability to ‘switch’ between different tasks. Our group has examined the cost associated with switching between a prosaccade (i.e., a standard task requiring a saccade to veridical target location) and an antisaccade (i.e., a non-standard task requiring a saccade mirror-symmetrical to veridical target) in predictable (i.e., AABB) and unpredictable (e.g., AABAB…) switching paradigms. Results have shown that reaction times (RTs) for a prosaccade preceded by an antisaccade (i.e., task-switch trial) are longer than when preceded by its same task-type (i.e., task-repeat trial), whereas RTs for antisaccade task-switch and task-repeat trials do not differ. The asymmetrical switch-cost has been attributed to an antisaccade task-set inertia that proactively delays a subsequent prosaccade (i.e., the unidirectional prosaccade switch-cost). A salient question arising from previous work is whether the antisaccade task-set inertia passively dissipates or persistently influences prosaccade RTs. Accordingly, participants completed separate AABB (i.e., A = prosaccade, B = antisaccade) task-switching conditions wherein the preparation interval for each trial was ‘short’ (1000–2000 ms; i.e., the timeframe used in previous work), ‘medium’ (3000–4000 ms) and ‘long’ (5000–6000 ms). Results demonstrated a reliable prosaccade switch-cost for each condition (ps < 0.02) and two one-sided test statistics indicated that switch cost magnitudes were within an equivalence boundary (ps < 0.05). Hence, null and equivalence tests demonstrate that an antisaccade task-set inertia does not passively dissipate and represents a temporally persistent feature of oculomotor control.
Similar content being viewed by others
Introduction
The educational, occupational and sport-related activities that we perform require efficient and effective alteration, or switching, between tasks. For example, driving a car requires that the operator rapidly alternate between breaking, throttle, and steering inputs while avoiding contact with other drivers. These complex tasks, and the ability to switch between them, are – in part – supported via the cognitive flexibility component of executive function (Miyake et al. 2000; Diamond 2013). An intriguing feature of cognitive flexibility is that the time required to switch between tasks can be asymmetrically influenced by the executive demands of the preceding task. In an initial demonstration of this phenomenon, Allport et al. (1994) had participants alternate between word-naming (i.e., standard task) and colour-naming (i.e., non-standard task) variants of the Stroop task using an AABB (i.e., A = word-naming, B = colour-naming) task-switching paradigm. Reaction times (RTs) for word-naming trials preceded by colour-naming trials (i.e., task-switch trials) were longer than word-naming trials preceded by their same task-type (i.e., task-repeat trial), whereas RTs for colour-naming task-switch and task-repeat trials did not differ. Allport et al. proposed that colour-naming required an executive mediated task-set that proactively delayed a subsequent word-naming trial (i.e., a switch-cost). In contrast, it was proposed that a word-naming trial does not elicit a switch cost because it is planned independent of an executive-mediated task-set (i.e., task-set inertia hypothesis). Subsequent functional magnetic resonance imaging and electroencephalographic studies have shown that RT switch-costs are related to increased and persistent task-related activity in the prefrontal cortex (PFC) (Evans et al. 2015; Brass and von Cramon 2004; Li et al. 2012; Yeung et al. 2006).
The majority of the task-switching literature has employed paradigms requiring concurrent executive and non-executive processes such as the Stroop task (i.e., language processing), face perception (i.e., emotive processing), target location/orientation (i.e., obligatory perception), and parity and consonant/vowel judgments (i.e., numerical and language processing). In contrast, our group (Weiler and Heath 2012a, b; 2014a, b) and others (Barton et al. 2006; Chan and DeSouza 2013; Manoach et al. 2007) have examined asymmetrical switch-costs via standard (i.e., prosaccade) and non-standard (i.e., antisaccade) oculomotor responses. The oculomotor paradigm provides a framework to examine switch-costs specific to top-down executive control (see details below) and provides the added benefit of examining task-switching for a response requiring concurrent spatiotemporal demands germane to goal-directed activities of daily living. In particular, prosaccades require that an individual “look” to a veridical target location and are mediated largely independent of top-down executive control (Pierrot-Deseilligny et al. 1995) via retinotopic projections from the superior colliculus (Wurtz and Albano 1980). In contrast, antisaccades are a non-standard task requiring a response mirror-symmetrical to a target. Antisaccades produce longer RTs (Hallett 1978) and less accurate and more variable endpoints (Dafoe et al. 2007; Gillen and Heath 2014a) than prosaccades and these behavioural ‘costs’ have been shown to reflect the executive demands of response suppression and vector inversion (i.e., 180º spatial transformation) (for reviews see Munoz and Everling 2004; Everling and Johnston 2013). More specifically, Everling and Johnston proposed that the PFC implements and maintains the neural activity and task rules, or task-set, governing antisaccade executive demands. Pro- and antisaccades performed in predictable (i.e., AABB; A = prosaccades, B = antisaccades) or unpredictable (e.g., ABBAAB…) trial sets exhibit a unidirectional prosaccade switch-cost wherein RTs for prosaccade task-switch trials are longer than their task-repeat counterparts, whereas RTs for antisaccades do not vary across task-switch and task-repeat trials (Weiler and Heath 2012a, b). The switch-cost is independent of the number of preceding antisaccades (Weiler et al. 2015), and the amplitude of the P300 event-related brain potential (ERP) for prosaccade task-switch trials is larger than counterpart task-repeat trials and equal in magnitude to antisaccade task-switch and task-repeat trials (Weiler et al. 2015). Hence, the unidirectional prosaccade switch-cost has been proposed to reflect an antisaccade task-set inertia that proactively delays a subsequent prosaccade.
A salient question in the task-switching literature is the persistence of a task-set inertia. One possibility is that an oculomotor task-set inertia is evanescent; that is, it passively decays and exerts a decreasing influence on response planning as the interval between iterative trials is increased. Indeed, Allport et al.’s (1994) original formulation asserted that proactive interference gradually decays to provide an automatic and necessary process to prevent the buildup of inefficient neural activity (see also Altmann 2002; Altmann and Gray 2008). To our knowledge, however, no direct evidence has been presented to support this view. An alternative account asserts that a switch-cost is composed of an active preparation and residual component. Active preparation refers to the cost that is reduced when participants are provided sufficient time to prepare for a new task, whereas a residual component is thought to reflect proactive interference that persists until a competing response is activated (i.e., task-set reconfiguration) (Meiran 1996; Rogers and Monsell 1995; see also Norman and Shallice 1986). In support of this view, Rogers and Monsell (1995) employed a predictable parity and vowel/consonant judgment switching task and reported a large switch-cost reduction (~ 100 ms) as the planning interval between task-switch trials increased from 150 to 600 ms, with no further reduction observed thereafter. Moreover, other parity and vowel/consonant switching studies have reported that a residual switch-costs persists for up to 3500 ms (Sohn et al. 2000; see also Schmitter-Edgecombe and Langill 2006). It is, however, unclear whether an evanescent or two-component decay influences the magnitude of an asymmetrical switch-cost (i.e., standard vs. non-standard tasks) and it is entirely unknown as to how long a task-set inertia persists in an oculomotor paradigm requiring selective executive control (i.e., pro- vs. antisaccades). To that end, we employed an AABB pro- and antisaccade paradigm in conditions (i.e., short, medium, and long) that manipulated the preparation interval between fixation onset and the cue signalling movement onset. In the ‘short’ preparation interval, a colour-coded fixation cross specifying the nature of an upcoming response (i.e., pro- vs. antisaccade) was presented for between 1000 and 2000 ms (i.e., the interval used in previous work by our group: e.g., Weiler and Heath 2012a, b, 2014a, b) prior to response cueing, whereas in the ‘medium’ and ‘long’ preparation intervals the fixation cross was presented between 3000–4000 ms and 5000–6000 ms prior to response cuing, respectively. In terms of research predictions, if the magnitude of the unidirectional prosaccade switch-cost decreases in relation to the length of the preparation interval then results would support the assertion of a passively decaying oculomotor task-set inertia. As a second possibility, if the switch-cost magnitude is largest for the short preparation interval followed by equivalent switch-costs for the medium and long preparation intervals then results would support the assertion that a rapidly dissipating active preparation and temporally durable residual component contributes to an oculomotor task-set inertia. As a third possibility, if the magnitude of the switch-costs is equivalent across each preparation interval then results would suggest that an oculomotor task-set inertia persists until a task-set reconfiguration.
Methods
Participants
Twenty participants were recruited from the University of Western Ontario community with sample size determined a priori based on the effect size derived from a paired-samples t-test contrasting task-switch and task-repeat prosaccade RTs (α = 0.05, power = 0.80, dz = 0.56) (Weiler and Heath 2012a). One participant was excluded from data analysis due to excessive signal loss (i.e., blinking due to dry eyes). Hence, the demographics for the 19 participants included in our analyses were 8 female and 11 male participants (18–26 years of age).
Participants self-reported normal or corrected-to-normal vision, right-hand dominance and indicated no previous neurological (including concussion) or neuropsychiatric conditions and indicated that they were free of current or previous diagnosis of SARS-CoV-2. Participants read a letter of information and gave informed written consent via a protocol approved by the Non-Medical Research Ethics Board, University of Western Ontario (ID: 114,975). This study was conducted according to the most recent iteration of the Declaration of Helsinki with the exception that participants were not registered in a database.
Apparatus and procedure
Participants sat on a height-adjustable chair in front of a table on which an LCD monitor (60 Hz, 8-ms response rate, 1280 × 960 pixels; Dell 3007WFP, Round Rock, TX) was located 550 mm from the table’s front edge. Participants placed their head in a head-chin rest, and the gaze location of their left eye was tracked via a video-based eye tracking system (EyeLink 1000 Plus; SR Research, Ottawa, ON, Canada) sampling at 1000 Hz. Prior to data collection, a nine-point calibration and validation of the viewing space was completed (i.e., < 1° of error). All visual events were controlled via MATLAB (R2018a; The MathWorks, Natick, MA) and the Psychophysics Toolbox extension (v. 3.0) (Brainard 1997; Kleiner et al. 2007) including the EyeLink Toolbox (Cornelissen et al. 2002). During data collection, the lights in the experimental suite were extinguished.
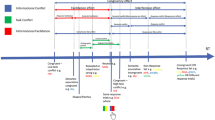
Visual stimuli were presented on a black screen (0.1 cd/m2) and included a midline-located red or green fixation cross (1° and luminance matched at 42 cd/m2) presented at participants’ eye level and targets (i.e., open white circle; 2.5° in diameter: 127 cd/m2) presented 13° (i.e., proximal target) and 17° (i.e., distal target) to the left and right of fixation and in the same horizontal plane. Fixation onset signalled participants to direct their gaze to its location and the colour of the fixation cross indicated the nature of an upcoming trial. For half of the participants, the green and red fixation cross indicated a pro- (i.e., saccade to veridical target location) or antisaccade (i.e., saccade to the target’s mirror-symmetrical location) response, respectively. For the other half of participants, the converse fixation-task colour mapping was used. Figure 1 shows that once a stable gaze was achieved (i.e., ± 1.5° for 450 ms) participants completed their response in each of three preparation intervals. In the first condition (i.e., short preparation interval), the attainment of a stable fixation gaze was followed by a uniformly distributed randomized foreperiod between 1000 and 2000 ms wherein the fixation cross remained visible to minimize memory load (Spector and Biederman 1976). Following the foreperiod, the fixation was extinguished and 200 ms thereafter a target was presented for 50 ms and cued participants to complete their response “quickly and accurately” (i.e., gap paradigm). The brief target presentation was used so that retinal feedback was unavailable in both pro- and antisaccade trials (Weiler and Heath 2012a, b). Following a trial, participants were instructed to move back to the perceived centre of a monitor after which time a new trial sequence (i.e., onset of the fixation cross) was initiated by the experimenter. In the other two conditions, the length of the preparation interval was increased to between 3000–4000 ms (i.e., medium preparation interval) and 5000–6000 ms (i.e., long preparation interval), respectively. The short preparation interval entails the same protocol as previous work by our group (Weiler and Heath 2012a, b; Weiler and Heath 2014a, b; Weiler et al. 2015; see also Tari et al. 2019; Tari and Heath 2019; Shukla et al. 2020; Shukla and Heath 2021), whereas the medium preparation interval reflects that used in a previous parity and consonant/vowel switching task (Meiran et al. 2000). In turn, the long preparation interval was used based on neuroimaging work demonstrating that the neural activity of an antisaccade persists for up to 6000 ms (Duschek et al. 2018). The range associated with each preparation interval (i.e., 1000 ms between lower- and upper-bound values) was necessary to prevent anticipation of response cueing. As well, it is important to recognize that the time between the end of a trial and onset of a subsequent trial could not be held constant due to individual, and trial-to-trial, differences in the time required to attain a stable gaze on the fixation cross. Instead, the current paradigm provided a framework for examining how the length of the preparation interval for a given trial influenced the unidirectional prosaccade switch-cost.
Schematic of the timeline of visual and movement events for the short (top panel), medium (middle panel), and long movement preparation intervals. For each condition a fixation cross was presented and following a stable gaze a movement preparation interval of short (1000–2000 ms), medium (3000–4000 ms) and long (5000–6000 ms) duration was initiated (see grey rectangles). Following the preparation interval, the fixation cross was extinguished and a target was presented 200 ms thereafter (i.e., gap paradigm). The onset of the target (small light grey square) served as the cue to pro- or antisaccade and target presentation was 50 ms in duration
For each preparation interval condition, pro- and antisaccades were arranged in an AABB paradigm (e.g., A = prosaccade, B = antisaccade) such that 40 prosaccade task-switch (i.e., prosaccade on trial N and an antisaccade on trial N-1) and 40 prosaccade task-repeat (e.g., prosaccade on trial N and N-1) trials were completed with an equivalent number of antisaccade task-switch and task-repeat trials (i.e., 160 trials/condition). As well, each condition contained an equal number of trials directed to each target location (i.e., left proximal, left distal, right proximal and right distal) ordered pseudo-randomly. The order of preparation interval conditions was randomized and completed in a single experimental session lasting approximately 70 min. Participants were provided a 10-min rest break between conditions. The first trial (i.e., pro- or antisaccade) in each condition was randomized and because such trials are neither a task-switch nor task-repeat trial, they were excluded from data analysis.
Data reduction, dependent variables, and statistical analysis
Gaze position data were filtered offline using a dual-pass Butterworth filter with a low-pass cut-off frequency of 15 Hz. A five-point central-finite difference algorithm was used to compute instantaneous velocities and acceleration. Saccade onset was determined when velocity and acceleration exceeded 30°/s and 8000°/s2, respectively. Saccade offset was determined when velocity fell below 30°/s for 40 ms. Trials involving a signal loss (e.g., an eye blink) were removed as were RTs less than 50 ms (Wenban-Smith and Findlay 1991) or greater than 2.5 standard deviations of a participant- and task-specific mean (Gillen and Heath 2014b). Less than 8% of total trials for any participant were omitted. Trials involving a directional error (i.e., a prosaccade instead of an instructed antisaccade or vice versa) were excluded from subsequent analysis because they are associated with planning mechanisms distinct from their directionally correct counterparts (DeSimone et al. 2014) and accounted for less than 8% of trials (i.e., 4% prosaccade and 12% antisaccades) and we note that the largest antisaccade error rate for any participant was 38%. Accordingly, our post-processed data-set provided a sufficient corpus to examine putative differences between pro- and antisaccade task-switch and task-repeat trials.
Dependent variables included RT (i.e., time from response cueing to saccade onset), interquartile range of RT (i.e., IQR of RT), saccade duration (i.e., time from saccade onset to saccade offset) and saccade gain variability (i.e., within-participant standard deviation of saccade amplitude/veridical target location). We report results for the IQR of RT to quantify whether between-condition differences in RT relate to condition-specific differences in the variability of saccade planning times. As well, the use of medians is advocated given a Fisher–Pearson coefficient (g1) greater than 0.75 (Doane and Seward 2011). In the present investigation the g1 value for RT was 1.07, whereas values for saccade duration and gain were less than 0.75. For that reason, median RT and mean saccade duration and gain variability were examined via 3 (condition: short, medium, and long movement preparation intervals) by 2 (task: pro-, antisaccade) by 2 (task-transition: task-switch, task-repeat) fully repeated measures ANOVA (α = 0.05). Where appropriate, Huynh–Feldt corrections for violations of sphericity are reported (i.e., degrees of freedom adjusted to one decimal place), and two one-sided test (TOST) statistics were used to determine whether means were within an equivalence boundary (Lakens 2017). Main effects and interactions were examined via power-polynomials (i.e., trend analysis) (Pedhazur 1997) or simple-effects (i.e., paired-samples t test).
Results
Reaction time
The main panels of Fig. 2a provide RT precent frequency histograms for pro- and antisaccade task-switch and task-repeat trials separately for each movement preparation condition. For each panel, the light and darker grey rectangles highlight anticipatory (i.e., < 100 ms) and short-latency (i.e., 100–150 ms) responses, respectively. As expected, prosaccades were associated with a greater percentage of anticipatory (3%) and short-latency (34%) responses than antisaccades (anticipatory: 3%; short-latency: 4%), and the main panels of Fig. 2a show that the percentage of anticipatory and short-latency prosaccades decreased across the short, medium, and long movement preparation intervals conditions. In addition, we contrasted task-switch and task-repeat trial RT distributions separately for pro- and antisaccade trials and separately for each preparation interval via the non-parametric Kolmogorov–Smirnov test. For prosaccades, RTs for task-switch and task-repeat trials were associated with different distributions for short (D = 2.11, p < 0.001), medium (D = 1.78, p = 0.004), and long (D = 1.95, p = 0.001) preparation intervals, whereas antisaccade task-switch and task-repeat trials were associated with comparable distributions (D = 0.63, 0.92, and 0.97 for short, medium, and long preparation intervals, respectively, ps = 0.82, 0.37, 0.31).
Panel A depicts reaction time (RT: ms) percent frequency distribution histograms for pro- and antisaccade task-switch and task-repeat trials in the short, medium and long movement preparation intervals. The light and dark grey rectangles denote anticipatory (i.e., < 100 ms) and short-latency (i.e., 100–150 ms) saccades, respectively. The histograms include trials involving inhibition failures and trials with RTs that exceeded 2.5 SDs of a participant- or task-specific mean. Only trials involving a directional error (i.e., a prosaccade instead of an instructed antisaccade or vice versa) or signal loss are excluded from the histograms. The presented mean depict RTs calculated before subsequent data post-processing. Panel B depicts post-processed participant-specific median RTs for pro- and antisaccade task-switch and task-repeat trials. Connecting lines emphasize the RT difference between task-switch and task-repeat trials for each participant. Black lines and error bars represent task-specific group means and associated 95% within-participant confidence intervals. Last, the inset panels depict group difference scores (i.e., task-switch minus task-repeat) for each task-type with error bars representing 95% between-participant confidence intervals. The absence of overlap between an error bar and zero indicates a reliable difference between task-switch and task-repeat trials
Parametric analysis of RT produced main effects of condition, F(1.2, 22.3) = 8.96, p = 0.004, ηp2 = 0.33, task, F(1, 18) = 38.74, p < 0.001, ηp2 = 0.68, and task-transition, F(1, 18) = 9.83, p = 0.006, ηp2 = 0.35, as well as a task by task-transition interaction, F(1, 18) = 4.66, p = 0.045, ηp2 = 0.21. RTs increased as a function of the short (256 ms, SD = 54), medium (275 ms, SD = 67) and long (307 ms, SD = 96) preparation intervals (significant linear effect: F(1, 18) = 9.64, p = 0.006, ηp2 = 0.35), and as expected, values for prosaccades (248 ms, SD = 65) were less than antisaccades (311 ms, SD = 76). In terms of the task by task-transition interaction, the inset panels of Fig. 2b show that RTs for prosaccade task-switch trials (259 ms, SD = 76) were longer than their task-repeat (238 ms, SD = 56) counterparts (t(18) = 3.54, p = 0.002, dz = 0.81), whereas RTs for antisaccade task-switch (312 ms, SD = 73) and task-repeat (309 ms, SD = 81) trials did not reliably differ (t(18) = 0.52, p = 0.61, dz = 0.12). Given the nature of our research objective, we note that the condition by task by task-transition interaction did not approach a conventional level of statistical significance, F(1.6, 29.0) = 0.18, p = 0.79, ηp2 = 0.01. Moreover, for prosaccades we computed participant-specific RT switch-costs (i.e., task-switch minus task-repeat) separately for each condition and compared each via TOST statistics. Results showed that difference scores for the short (18 ms, SD = 21), medium (20 ms, SD = 36) and long (24 ms, SD = 37) preparation intervals were within an equivalence boundary (all ts(18) < 1.70, ps < 0.05). In other words, the magnitude of the switch-cost was equivalent across each preparation interval.
RT IQR yielded a main effect of task-transition, F(1, 18) = 7.68, p = 0.01, ηp2 = 0.30, and a task by task-transition interaction, F(1, 18) = 5.81, p = 0.03, ηp2 = 0.24. IQRs for prosaccade task-switch trials (109 ms, SD = 68) were larger than their task-repeat counterparts (78 ms, SD = 35) (t(18) = 3.37, p = 0.003, dz = 0.77), whereas values for antisaccade task-switch (94 ms, SD = 57) and task-repeat (88 ms, SD = 49) trials did not reliably differ (t(18) = 0.73, p = 0.48, dz = 0.17). RT IQR did not yield a reliable main effect of condition, F(1.5, 26.1) = 0.10, p = 0.85, dz = 0.005, nor any high-order interaction involving condition, all F(2, 36) < 0.51, p > 0.61, ηp2 < 0.03.
Saccade duration and gain variability produced main effects of task, F(1, 18) = 8.45 and 86.32, p = 0.009 and < 0.001, ηp2 = 0.32 and 0.83: prosaccades were shorter in duration (MT: 71 ms, SD = 6) and less variable (0.11, SD = 0.05) than antisaccades (MT: 81 ms, SD = 14, gain variability: 0.21, SD = 0.05) (Fig. 3). Neither saccade duration nor gain variability produced a main effect for condition nor any higher-order interaction involving condition, all F(1.4, 24.3 and 2, 36) < 3.14 and < 2.57, ps > 0.08 and > 0.09, all ηp2 < 0.15 and < 0.13.
Discussion
We employed a predictable AABB pro- and antisaccade task-switching paradigm across conditions that manipulated the preparation interval between fixation onset and response cueing to determine whether an antisaccade task-set inertia exhibits a monotonic or non-monotonic decay or persists until the activation of a prosaccade. Before outlining our primary research objective, we first discuss the general impact of task-type (i.e., pro- and antisaccades) and movement planning intervals.
Pro- and antisaccades: the executive demands of response suppression and vector inversion increase RT
Prosaccade RTs and saccade durations were shorter, and endpoints less variable than antisaccades, and was a finding independent of task-transition (i.e., task-switch and task-repeat trials) and movement preparation intervals. The RT findings evince prosaccade mediation largely independent of top-down executive control via direct retinotopic projections within the superior colliculus (Wurtz and Albano 1980). The longer antisaccade RTs reflect the executive demands of implementing a non-standard task-set supporting the inhibition of a prosaccade (i.e., response suppression) and decoupling the spatial relations between stimulus and response (i.e., vector inversion) (for reviews see, Everling and Johnston 2013; Munoz and Everling 2004). As well, that antisaccades produced longer saccade durations and more variable endpoints has been shown to reflect that the absence of a visual target at the movement goal decreases saccade burst neuron activity in the superior colliculus and frontal eye fields (Edelman and Goldberg 2001; Edelman et al. 2006) and renders movement planning via visual information (i.e., relative) functionally distinct from the absolute retinotopic coordinates supporting prosaccades (Dafoe et al. 2007; Gillen and Heath 2014a, b).
Fixation to response cueing interval influences saccade RTs
The intervals between fixation onset and response cueing (i.e., preparation interval) were short (i.e., 1000–2000 ms), medium (i.e., 3000–4000 ms) and long (i.e., 5000–6000 ms) in duration. Results showed that RTs increased linearly in relation to increasing preparation interval and was a finding independent of task and task-transition. In accounting for this finding, Posner and Boies (1971) reported that peak alertness in responding to a stimulus occurs approximately 500 ms following a preparatory cue and an extensive literature reports that task-based vigilance, and the ability to rapidly respond to an exogenous stimulus, decreases in relation to the length of a sustained attention interval (for reviews see, Fortenbaugh et al. 2017; Sarter et al. 2001). From a behavioural perspective, the resource-control theory asserts that a default network of self-generated thought (so-called mind wandering) outcompetes task-based vigilance and increases RT in relation to the length of a sustained attention interval (Thomson et al. 2015; Smallwood 2013). Moreover, in the oculomotor control literature, single-cell recordings in non-human primates have shown that extended discharge activity of fixation neurons in the superior colliculus delays the accumulation (for review see, Krauzlis et al. 2017; see also Pouget et al. 2011) of saccade burst neuron activity (Khanna et al. 2019; Zhou and Constantinidis 2017) – a process that gives rise to an increase in RT. Hence, the resource-control theory combined with the electrophysiological operating principles of the oculomotor system can account for the observed finding that RTs increased with the length of the movement preparation interval. Alternatively, recent work has proposed that theta-dependent shifts in the visuo-attentive system result in alternating periods of enhanced or diminished perceptual sensitivity that can influence oculomotor reactivity (Fiebelkorn and Kastner 2019; Senoussi et al. 2019). Given such oscillations (i.e., 4 Hz), it is possible that the increase in RT across the short, medium and long movement preparation intervals reflect that an increasing preparation interval results in an increased opportunity for “attentional shifts” away from a task-dependent goal and thus increases saccade planning times.
Short, medium and long preparation intervals do not influence the magnitude of the unidirectional prosaccade switch-cost
RTs for prosaccade task-switch trials were on average 22 ms (SD = 25) longer than their task-repeat counterparts, whereas values for antisaccade task-switch and task-repeat trials did not reliably differ, a result consistent across the short, medium, and long preparation intervals. The magnitude of this unidirectional prosaccade switch-cost is comparable to the pooled average switch-cost (24 ms, CI95% = 11) reported elsewhere (Chan and DeSouza 2013; Heath et al. 2015; Manoach et a. 2007; Weiler and Heath 2012a, b, 2014a, b; Weiler et al. 2015). In accounting for this finding, some work has proposed that an AABB paradigm does not provide a valid framework for evaluating switch-costs given that the second of two consecutively completed tasks may produce a repetition benefit (Wylie and Allport 2000); that is, the observed difference between prosaccade task-switch and task-repetition trials may reflect a RT facilitation for the latter trial-type. Notably, however, RTs for task-repeat prosaccades are equivalent to counterparts completed in a separate block of trials (Weiler and Heath 2014b; Tari et al. 2019), and Stroop (Allport and Wylie 1999) and parity and consonant/vowel judgment (Schmidt and Liefooghe 2016; Schmitz and Voss 2014) paradigms concluded that RT differences between task-switch and task-repeat trials reflects a switch cost for the former trial-type. Accordingly, and as per previous work, we propose that a task-set inertia – and not repetition priming– accounts for the unidirectional prosaccade switch-cost.
The unidirectional prosaccade switch-cost was comparable across short (19 ms, SD = 21), medium (20 ms, SD = 36) and long (24 ms, SD = 37) preparation intervals and is a conclusion supported by null hypothesis and equivalence testing. This finding indicates that an antisaccade task-set does not passively decay with time (e.g., Allport et al. 1994). If that were the case, then an increase in the movement preparation interval would be expected to produce a monotonic decrease in the magnitude of the unidirectional prosaccade switch-cost. In addition, the present results provide no evidence that the switch-cost is composed of a short-lasting movement preparation component and longer lasting residual component. As indicated in the Introduction, a two-component decay account would produce a non-monotonic switch-cost reduction such that a large switch-cost reduction would be observed between the short and medium preparation intervals with a smaller (or null) reduction between the medium and long preparation intervals. The absence of a non-monotonic account cannot be related to a movement preparation interval of insufficient length as the long preparation interval (i.e., 5000–6000 ms) is greater than that used in task-switching studies (maximum 3500 ms) involving alternating parity and consonant/vowel judgments (Meiran 1996; Rogers and Monsell 1995). Hence, an important question to address is why the present work reported an equivalent magnitude switch cost for each movement preparation interval. A parsimonious account is that previous work contrasted tasks that did not differ in terms of difficulty (i.e., parity and consonant/vowel judgments) and hence did not elicit asymmetrical switch-costs (e.g., Meiran 1996; Rogers and Monsell 1995; Sohn et al. 2000). Indeed, it may be the case that the computational demands of evoking a non-standard task renders a temporally persistent task-set inertia than paradigms that involve alternating between tasks of comparable complexity. This view is bolstered by evidence that antisaccades are computationally more complex than prosaccades and require an extensive frontoparietal network to instantiate an executive-mediated task-set (Everling and Johnston 2013; Munoz and Everling 2004). Moreover, Duschek et al. (2018) examined cerebral blood flow modulation during the preparatory and post-movement intervals of pro- and antisaccades via functional transcranial Doppler sonography (fTCD). fTCD is an ultrasonic technique that measures fast and continuous changes in cerebral artery blood flow velocities with an increase in velocity attributed to a task-dependent increase in neural activity. Duschek et al. noted that the preparatory interval of antisaccades produced an increase in blood flow velocity through the middle cerebral artery and that this change persisted for up to 6000 ms following antisaccade completion. Given that the PFC is within the perfusion territory of the middle cerebral artery it was concluded that the increase in cerebral blood flow represents “[…] the complexity of the upcoming task demands as well as proactive interference” (p. 65). A more recent fTCD study contrasting stimulus-driven (i.e., saccade at target onset) and minimally delayed (i.e., saccade when a target is extinguished, and a task requiring response suppression) saccades provides parallel evidence that a non-standard oculomotor task-set results in a persistent increase in perfusion to the PFC (Tari et al. 2021). As such, we propose that the equivalent magnitude unidirectional prosaccade switch-cost across short, medium and long preparation intervals reflects proactive inhibition arising from a temporally persistent antisaccade task-set inertia.
An important issue to address is the environment required to “release” the proactive inhibition of an antisaccade task-set inertia (e.g., release of proactive interference; see Brown 1958; Peterson and Peterson 1959). Kliegl and Bäuml’s (2021) recent review acknowledges that no imaging studies have directly examined the neural underpinnings associated with a release of proactive inhibition/interference across increasing movement preparation intervals. That said, Rogers and Monsell’s (1995) task-set reconfiguration hypothesis asserts that the completion of a non-standard task increases top-down executive control and that the goal-state for this non-standard response may persist until a response is adopted entailing exogenous (or standard) stimulus–response relations (e.g., prosaccade). In other words, the executive-mediated activity associated with an antisaccade task-set may persist – within a reasonable timeframe – until a task-set reconfiguration via the evocation of prosaccade (see also Mayr 2002). Future work by our group seeks to directly examine this issue via examining the event-related amplitude of the P300 as a function of increasing movement preparation intervals. Addressing this issue in future work would provide theoretical predictions for the persistence of the task-set inertia documented in Stroop word- and colour-naming tasks (e.g., Allport et al. 1994) and provide a basis for understanding asymmetrical task-switch costs in educational, occupational and sport domains. As a more practical example, a better understanding of asymmetrical switch-costs could support driving management and allow motor vehicle operators to better understand the costs and consequences of switching between a non-standard driving task (e.g., managing an instrument warning light) with a highly frequent and standard one (e.g., providing steering wheel input).
Conclusions
The unidirectional prosaccade switch-cost did not exhibit a monotonic or non-monotonic reduction across movement preparation intervals ranging from 1000 to 6000 ms. We believe that such results add importantly to the literature insomuch as they demonstrate that an antisaccade task-set persists inertially for up to 6000 ms and actively interferes with the planning of a prosaccade.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Allport A, Wylie G (1999) Task-switching: positive and negative priming of task-set. In: Humphreys GW, Duncan J, Treisman A (eds) Attention, space, and action: studies in cognitive neuroscience. Oxford University Press, Oxford, pp 273–296
Allport DA, Styles EA, Hsieh S (1994) Shifting intentional set: exploring the dynamic control of tasks. In: Umiltà C, Moscovitch M (eds) Attention and performance 15: conscious and nonconscious information processing. The MIT Press, Cambridge, pp 421–452
Altmann EM (2002) Functional decay of memory for tasks. Psychol Res 66:287–297. https://doi.org/10.1007/s00426-002-0102-9
Altmann EM, Gray WD (2008) An integrated model of cognitive control in task switching. Psychol Rev 115:602–639. https://doi.org/10.1037/0033-295X.115.3.602
Barton JJ, Raoof M, Jameel O, Manoach DS (2006) Task-switching with antisaccades versus no-go trials: a comparison of inter-trial effects. Exp Brain Res 172:114–119. https://doi.org/10.1007/s00221-005-0313-6
Brainard DH (1997) The psychophysics toolbox. Spat vis 10:433–436
Brass M, von Cramon DY (2004) Decomposing components of task preparation with functional magnetic resonance imaging. J Cogn Neurosci 16:609–620. https://doi.org/10.1162/089892904323057335
Brown J (1958) Some tests of the decay theory of immediate memory. Q J Exp Psychol 10:12–21. https://doi.org/10.1080/17470215808416249
Chan JL, DeSouza JF (2013) The effects of attentional load on saccadic task switching. Exp Brain Res 227:301–309. https://doi.org/10.1007/s00221-013-3452-1
Cornelissen F, Peters E, Palmer J (2002) The eyelink toolbox: eye tracking with MATLAB and the psychophysics toolbox. Behav Res Methods Instrum Comput 34:613–617. https://doi.org/10.3758/bf03195489
Dafoe JM, Armstrong IT, Munoz DP (2007) The influence of stimulus direction and eccentricity on pro- and anti-saccades in humans. Exp Brain Res 179:563–570. https://doi.org/10.1007/s00221-006-0817-8
DeSimone JC, Weiler J, Aber GS, Heath M (2014) The unidirectional prosaccade switch-cost: correct and error antisaccades differentially influence the planning times for subsequent prosaccades. Vis Res 96C:17–24. https://doi.org/10.1016/j.visres.2013.12.005
Diamond A (2013) Executive functions. Annu Rev Psychol 64:135–168. https://doi.org/10.1146/annurev-psych-113011-143750
Doane DP, Seward LE (2011) Measuring skewness: a forgotten statistic. J Statistics Education 19:1–18. https://doi.org/10.1080/10691898.2011.11889611
Duschek S, Hoffmann A, Montoro CI, Reyes Del Paso GA, Schuepbach D, Ettinger U (2018) Cerebral blood flow modulations during preparatory attention and proactive inhibition. Biol Psychol 137:65–72. https://doi.org/10.1016/j.biopsycho.2018.07.003
Edelman JA, Goldberg ME (2001) Dependence of saccade-related activity in the primate superior colliculus on visual target presence. J Neurophysiol 86:676–691. https://doi.org/10.1152/jn.2001.86.2.676
Edelman JA, Valenzuela N, Barton JJ (2006) Antisaccade velocity, but not latency, results from a lack of saccade visual guidance. Vision Res 46:1411–1421. https://doi.org/10.1016/j.visres.2005.09.013
Evans LH, Herron JE, Wilding EL (2015) Direct real-time neural evidence for task-set inertia. Psychol Sci 26:284–290. https://doi.org/10.1177/0956797614561799
Everling S, Johnston K (2013) Control of the superior colliculus by the lateral prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 368:20130068. https://doi.org/10.1098/rstb.2013.0068
Fiebelkorn IC, Kastner S (2019) A rhythmic theory of attention. Trends Cogn Sci 23:87–101. https://doi.org/10.1016/j.tics.2018.11.009
Fortenbaugh FC, DeGutis J, Esterman M (2017) Recent theoretical, neural, and clinical advances in sustained attention research. Ann N Y Acad Sci 1396:70–91. https://doi.org/10.1111/nyas.13318
Gillen C, Heath M (2014a) Target frequency influences antisaccade endpoint bias: evidence for perceptual averaging. Vis Res 105:151–158. https://doi.org/10.1016/j.visres.2014.10.010
Gillen C, Heath M (2014b) Perceptual averaging governs antisaccade endpoint bias. Exp Brain Res 232:3201–3210. https://doi.org/10.1007/s00221-014-4010-1
Hallett PE (1978) Primary and secondary saccades to goals defined by instructions. Vis Res 8:1279–1296. https://doi.org/10.1016/0042-6989(78)90218-3
Heath M, Hassall CD, MacLean S, Krigolson OE (2015) Event-related brain potentials during the visuomotor mental rotation task: the contingent negative variation scales to angle of rotation. Neuroscience 311:153–165. https://doi.org/10.1016/j.neuroscience.2015.10.018
Khanna SB, Snyder AC, Smith MA (2019) Distinct sources of variability affect eye movement preparation. J Neurosci 39:4511–4526. https://doi.org/10.1523/JNEUROSCI.2329-18.2019
Kleiner M, Brainard D, Pelli D, Ingling A, Murray R, Broussard C (2007) What’s new in Psychtoolbox-3. Perception 36:1–16
Kliegl O, Bäuml KT (2021) The mechanisms underlying interference and inhibition: a review of current behavioral and neuroimaging research. Brain Sci 11:1246. https://doi.org/10.3390/brainsci11091246
Krauzlis RJ, Goffart L, Hafed ZM (2017) Neuronal control of fixation and fixational eye movements. Philos Trans R Soc Lond B Biol Sci 372:20160205. https://doi.org/10.1098/rstb.2016.0205
Lakens D (2017) Equivalence tests: a practical primer for t-tests, correlations, and meta-analyses. Soc Psychol Personal Sci 8:355–436. https://doi.org/10.1177/1948550617697177
Li L, Wang M, Zhao QJ, Fogelson N (2012) Neural mechanisms underlying the cost of task switching: an ERP study. PLoS ONE 7:e42233. https://doi.org/10.1371/journal.pone.0042233
Manoach DS, Thakkar KN, Cain MS, Polli FE, Edelman JA, Fischl B, Barton JJ (2007) Neural activity is modulated by trial history: a functional magnetic resonance imaging study of the effects of a previous antisaccade. J Neurosci 27:1791–1798. https://doi.org/10.1523/jneurosci.3662-06.2007
Mayr U (2002) Inhibition of action rules. Psychon Bull Rev 9:93–99. https://doi.org/10.3758/bf03196261
Meiran N (1996) Reconfiguration of processing mode prior to task performance. J Exp Psychol Learn Mem Cogn 22:1423–1442. https://doi.org/10.1037/0278-7393.22.6.1423
Meiran N, Chorev Z, Sapir A (2000) Component processes in task switching. Cogn Psychol 41:211–253. https://doi.org/10.1006/cogp.2000.0736
Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000) The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol 41:49–100. https://doi.org/10.1006/cogp.1999.0734
Munoz DP, Everling S (2004) Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci 5:218–228. https://doi.org/10.1038/nrn1345
Norman DA, Shallice T (1986) Attention to action: willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D (eds) Consciousness and self-regulation. Plenum, New York, pp 1–18
Pedhazur EJ (1997) Multiple regression in behavioral research. Thomson Learning Inc., Toronto
PetersonPeterson LMJ (1959) Short-term retention of individual verbal items. J Exp Psychol 58:193–198. https://doi.org/10.1037/h0049234
Pierrot-Deseilligny C, Rivaud S, Gaymard B, Müri R, Vermersch AI (1995) Cortical control of saccades. Ann Neurol 37:557–567. https://doi.org/10.1002/ana.410370504
Posner MI, Boies SJ (1971) Components of attention. Psychol Rev 78:391–408. https://doi.org/10.1037/h0031333
Pouget P, Logan GD, Palmeri TJ, Boucher L, Paré M, Schall JD (2011) Neural basis of adaptive response time adjustment during saccade countermanding. J Neurosci 31:12604–12612. https://doi.org/10.1523/JNEUROSCI.1868-11.2011
Rogers RD, Monsell S (1995) Costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen 124:207–231. https://doi.org/10.1037/0096-3445.124.2.207
Sarter M, Givens B, Bruno JP (2001) The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev 35:146–160. https://doi.org/10.1016/s0165-0173(01)00044-3
Schmidt JR, Liefooghe B (2016) Feature integration and task switching: diminished switch costs after controlling for stimulus, response, and cue repetitions. PLoS ONE 11:e0151188. https://doi.org/10.1371/journal.pone.0151188
Schmitter-Edgecombe M, Langill M (2006) Costs of a predictable switch between simple cognitive tasks following severe closed-head injury. Neuropsychology 20:675–684. https://doi.org/10.1037/0894-4105.20.6.675
Schmitz F, Voss A (2014) Components of task switching: a closer look at task switching and cue switching. Acta Psychol (Amst) 151:184–196. https://doi.org/10.1016/j.actpsy.2014.06.009
Senoussi M, Moreland JC, Busch NA, Dugué L (2019) Attention explores space periodically at the theta frequency. J vis 19:1–17. https://doi.org/10.1167/19.5.22
Shukla D, Heath M (2021) A single bout of exercise provides a persistent benefit to cognitive flexibility. Res Q Exerc Sport. https://doi.org/10.1080/02701367.2021.1873902
Shukla D, Al-Shamil Z, Belfry G, Heath M (2020) A single bout of moderate intensity exercise improves cognitive flexibility: evidence from task-switching. Exp Brain Res 238:2333–2346. https://doi.org/10.1007/s00221-020-05885-w
Smallwood J (2013) Distinguishing how from why the mind wanders: a process-occurrence framework for self-generated mental activity. Psychol Bull 139:519–535. https://doi.org/10.1037/a0030010
Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS (2000) The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97:13448–13453. https://doi.org/10.1073/pnas.240460497
Spector A, Biederman I (1976) Mental set and mental shift revisited. Am J Psychol 89:669–679. https://doi.org/10.2307/1421465
Tari B, Heath M (2019) Pro- and antisaccade task-switching: response suppression-and not vector inversion-contributes to a task-set inertia. Exp Brain Res 237:3475–3484. https://doi.org/10.1007/s00221-019-05686-w
Tari B, Fadel MA, Heath M (2019) Response suppression produces a switch-cost for spatially compatible saccades. Exp Brain Res 237:1195–1203. https://doi.org/10.1007/s00221-019-05497-z
Tari B, Shirzad M, Badcock NA, Belfry GR, Heath M (2021) “Delaying” a saccade: preparatory phase cortical hemodynamics evince the neural cost of response inhibition. Brain Cogn 154:105808. https://doi.org/10.1016/j.bandc.2021.105808
Thomson DR, Smilek D, Besner D (2015) Reducing the vigilance decrement: the effects of perceptual variability. Conscious Cogn 33:386–397. https://doi.org/10.1016/j.concog.2015.02.010
Weiler J, Heath M (2012a) Task-switching in oculomotor control: unidirectional switch-cost when alternating between pro- and antisaccades. Neurosci Lett 530:150–154. https://doi.org/10.1016/j.neulet.2012.10.007
Weiler J, Heath M (2012b) The prior-antisaccade effect influences the planning and online control of prosaccades. Exp Brain Res 216:545–552. https://doi.org/10.1007/s00221-011-2958-7
Weiler J, Heath M (2014a) Oculomotor task switching: alternating from a nonstandard to a standard response yields the unidirectional prosaccade switch-cost. J Neurophysiol 112:2176–2184. https://doi.org/10.1152/jn.00352.2014
Weiler J, Heath M (2014b) Repetitive antisaccade execution does not increase the unidirectional prosaccade switch-cost. Acta Psychol (Amst) 146:67–72. https://doi.org/10.1016/j.actpsy.2013.12.005
Weiler J, Hassall CD, Krigolson OE, Heath M (2015) The unidirectional prosaccade switch-cost: electroencephalographic evidence of task-set inertia in oculomotor control. Behav Brain Res 278:323–329. https://doi.org/10.1016/j.bbr.2014.10.012
Wenban-Smith MG, Findlay JM (1991) Express saccades: is there a separate population in humans? Exp Brain Res 87:218–222. https://doi.org/10.1007/bf00228523
Wurtz RH, Albano JE (1980) Visual-motor function of the primate superior colliculus. Annu Rev Neurosci 3:189–226. https://doi.org/10.1146/annurev.ne.03.030180.001201
Wylie G, Allport A (2000) Task switching and the measurement of “switch costs.” Psychol Res 63:212–233. https://doi.org/10.1007/s004269900003
Yeung N, Nystrom LE, Aronson JA, Cohen JD (2006) Between-task competition and cognitive control in task switching. J Neurosci 26:1429–1438. https://doi.org/10.1523/jneurosci.3109-05.2006
Zhou X, Constantinidis C (2017) Fixation target representation in prefrontal cortex during the antisaccade task. J Neurophysiol 117:2152–2162. https://doi.org/10.1152/jn.00908.2016
Funding
This work is supported by a Discovery Grant and Postgraduate Doctoral Scholarship from the Natural Sciences and Engineering Research Council (NSERC) of Canada, and Faculty Scholar and Major Academic Development Fund Awards from the University of Western Ontario.
Author information
Authors and Affiliations
Contributions
BT and MH conceived and designed the research, BT, CE, PP and CD performed experiments; BT, CE, PP, CD and MH analyzed data; BT and MH interpreted results of experiments; BT and MH prepared figures; BT and MH drafted the manuscript; BT and MH edited and revised the manuscript; BT, CE, PP, CD and MH approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
This work was approved by the Non-Medical Research Ethics Board, University of Western Ontario (ID: 114975) and was conducted according to the most recent iteration of the Declaration of Helsinki.
Consent to participate
Participants read a letter of information and gave informed written consent.
Consent to publish
NA.
Additional information
Communicated by Bill J Yates.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tari, B., Edgar, C., Persaud, P. et al. The unidirectional prosaccade switch-cost: no evidence for the passive dissipation of an oculomotor task-set inertia. Exp Brain Res 240, 2061–2071 (2022). https://doi.org/10.1007/s00221-022-06394-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-022-06394-8