Abstract
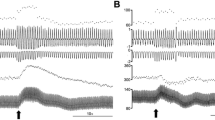
Experiments were done to investigate the role of glutamatergic systems in the median preoptic nucleus (MnPO) in the water ingestion induced by administration of angiotensin II (ANG II) in the subfornical organ (SFO) in the awake rat. Microdialysis methods were utilized to quantify the extracellular content of glutamate (Glu) in the region of MnPO. Microinjection of ANG II (10−10 M) into the SFO significantly increased the release of Glu in the MnPO in the rats under the condition that water is available for drinking and the rats under the condition that water is not available for drinking. The amount of initial maximal increases in the Glu levels elicited by the ANG II injection was quite similar in drinking and non-drinking rats, whereas the duration of the response was much longer in non-drinking than in drinking rats. The amount of water ingestion in 20 min immediately after the ANG II injection was significantly enhanced by previous injections of N-methyl-d-aspartate (NMDA, 10 μM) into the MnPO, while the ANG II-induced water ingestion was attenuated by pretreatment with the NMDA antagonist dizocilpine (MK-801, 10 μM). The amount of water intake elicited by the ANG II injection into the SFO was enhanced by previous injections of either the non-NMDA agonist kainic acid (KA, 50 μM) or quisqualic acid (QA, 50 μM) into the MnPO. On the contrary, the ANG II-induced drinking response was diminished by pretreatment with the non-NMDA antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM) in the MnPO. Each injection of NMDA, KA, and QA into the MnPO produced drinking behavior. These results imply that the glutamatergic neural pathways to the MnPO may transmit the information for eliciting drinking in response to ANG II acting at the SFO. Our data further provide evidence that the ANG II-induced dipsogenic response may be mediated through both NMDA and non-NMDA glutamatergic receptor mechanisms in the MnPO.




Similar content being viewed by others
References
Bellin SL, Landas SK, Johnson AK (1988) Selective catecholamine depletion of structures along the ventral lamina terminalis: effects on experimentally-induced drinking and pressor responses. Brain Res 456:9–16
Cunningham JT, Johnson AK (1989) Decreased norepinephrine in the ventral lamina terminalis region is associated with angiotensin II-induced drinking response deficits following local 6-hydroxydopamine injections. Brain Res 480:65–71
Cunningham JT, Johnson AK (1991) The effects of central norepinephrine infusions on drinking behavior induced by angiotensin after 6-hydroxydopamine injections into the anteroventral region of the third ventricle (AV3 V). Brain Res Bull 558:112–116
Duan P-G, Kawano H, Masuko S (2008) Collateral projections from the subfornical organ to the median preoptic nucleus and paraventricular hypothalamic nucleus in the rat. Brain Res 1198:68–72
Gutmann MB, Jones DL, Ciriello J (1988) Effect of paraventricular nucleus lesions on drinking and pressor responses to ANG II. Am J Physiol 255:R882–R887
Kawano H, Masuko S (1993) Synaptic inputs of neuropeptide Y immunoreactive noradrenergic nerve terminals to neurons in the nucleus medians which project to the paraventricular nucleus of the rat: a combined immunohistochemical and retrograde tracing methods. Brain Res 600:74–80
Koiaj M, Renaud LP (2010) Metabotropic glutamate receptors in the median preoptic neurons modulate neuronal excitability and glutamatergic and GABAergic inputs from the subfornical organ. J Neurophysiol 103:1104–1112
Lind RW, Johnson AK (1982) Subfornical organ-median preoptic connections and drinking and pressor responses to angiotensin II. J Neurosci 2:1043–1051
Lind RW, Swanson LW, Ganten D (1984) Angiotensin II immunoreactivity in neural afferents and efferents of the subfornical organ of the rat. Brain Res 321:209–215
Lind RW, Swanson LW, Ganten D (1985) Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system: an immunohistochemical study. Neuroendocrinology 60:2–24
Mangiapane ML, Simpson JB (1980) Subfornical organ: forebrain site of pressor and dipsogenic action of angiotensin II. Am J Physiol 239:R382–R389
McKinley MJ, McAllen RM, Mendelsohn FAO, Allen AM, Chai SY, Oldfield BJ (1990) Circumventricular organ: neuroendocrine interfaces between the brain and the hemal milieu. Front Neuroendocrinol 11:91–127
Miselis RR (1981) The efferent projections of the subfornical organ of the rat: a circumventricular organ within a neural network subserving water balance. Brain Res 23:1–23
Miyakubo H, Yamamoto K, Hatakenaka S, Hayashi Y, Tanaka J (2003) Drinking decreases the noradrenaline release in the median preoptic area caused by hypovolemia in the rat. Behav Brain Res 145:1–5t
Saper CB, Levisohn D (1983) Afferent connections of median preoptic nucleus in the rat: anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3 V) region. Brain Res 288:21–31
Schwats JA, Reilly NS (2008) Angiotensin and NMDA receptors in the median preoptic nucleus mediate hemodynamic response patterns to stress. Am J Regul Integr Comp Physiol 295:R155–R165
Takahashi M, Tanaka J (2017) GABAergic modulation of noradrenaline release caused by blood pressure changes in the rat median preoptic area. NeuroReport 28:485–491
Takahashi M, Hayashi Y, Tanaka J (2017) Glutamatergic modulation of noradrenaline release in the rat median preoptic area. Brain Res Bull 130:36–41
Tanaka J (1989) Involvement of median preoptic nucleus in the regulation of paraventricular vasopressin neurons by the subfornical organ in the rat. Exp Brain Res 76:47–54
Tanaka J (2002) α-Adrenergic control of ANG II-induced drinking in the rat median preoptic nucleus. Physiol Behav 77:155–160
Tanaka J, Nomura M (1993) Involvement of neurons sensitive to angiotensin II in the median preoptic nucleus in the drinking response induced by angiotensin II activation of the subfornical organ in rats. Exp Neurol 119:235–239
Tanaka J, Hori K, Koito H, Nomura M (1992) Enhanced monoamine release in the median preoptic area following reduced extracellular fluid volume in rats. Neurosci Lett 147:110–113
Tanaka J, Hayashi Y, Shimamune S, Hori K, Nomura M (1997) Subfornical organ efferents enhance extracellular noradrenaline concentrations in the median preoptic area in rats. Neurosci Lett 230:171–174
Tanaka J, Miyakubo H, Fujisawa S, Nomura M (2003) Reduced dipsogenic response induced by angiotensin II activation of the subfornical organ projections to the median preoptic nucleus in estrogen-treated rats. Exp Neurol 179:83–89
Ushigome A, Nomura M, Tanaka J (2004) Modulation of noradrenaline release in the median preoptic area by GABAergic inputs from the organum vasculosum of the lamina terminalis in the rat. Neurochem Int 44:139–144
Wilkin LD, Patel KP, Schmid PG, Johnson AK (1987) Increased norepinephrine turnover in the median preoptic nucleus following reduced extracellular fluid volume. Brain Res 423:369–372
Xu Z, Herbert J (1998) Effects of intracerebroventricular dizocilpine (MK801) on dehydration-induced dipsogenic responses, plasma vasopressin and c-fos expression in the rat forebrain. Brain Res 784:91–99
Xu Z, Lane JM, Zhu B, Herbert J (1997) Dizocilpine maleate, an N-metyl-D-aspartate antagonist, inhibits dipsogenic responses and c-fos expression induced by intracerebral infusion of angiotensin II. Neuroscience 27:203–214
Acknowledgements
This research was supported in part by JSPS KAKENHI Grant 18K02442.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Thomas Deller.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ushigome, A., Momoi, K., Takahashi, M. et al. Involvement of glutamatergic mechanisms in the median preoptic nucleus in the dipsogenic response induced by angiotensinergic activation of the subfornical organ in rats. Exp Brain Res 238, 73–80 (2020). https://doi.org/10.1007/s00221-019-05681-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-019-05681-1