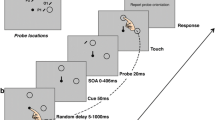
Abstract
The present study investigated the effect of auditory feedback on planning and control of two-segment reaching movements and eye–hand coordination. In particular, it was examined whether additional auditory information indicating the progression of the initial reach (i.e., passing the midway and contacting the target) affects the performance of that reach and gaze shift to the second target at the transition between two segments. Young adults performed a rapid two-segment reaching task, in which both the first and second segments had two target sizes. One out of three auditory feedback conditions included the reach-progression information: a continuous tone was delivered at a consistent timing during the initial reach from the midway to the target contact. Conversely, the other two were control conditions: a continuous tone was delivered at a random timing in one condition or not delivered in the other. The results showed that the initial reach became more accurate with the auditory reach-progression cue compared to without any auditory cue. When that cue was available, movement time of the initial reach was decreased, which was accompanied by an increased peak velocity and a decreased time to peak velocity. These findings suggest that the auditory reach-progression feedback enhanced the preplanned control of the initial reach. Deceleration time of that reach was also decreased with auditory feedback, but it was observed regardless of whether the sound contained the reach-progression information. At the transition between the two segments, the onset latencies of both the gaze shift and reach to the second target became shorter with the auditory reach-progression cue, the effect of which was pronounced when the initial reach had a higher terminal accuracy constraint. This suggests that the reach-progression cue enhanced verification of the termination of initial reach, thereby facilitating the initiation of eye and hand movements to the second target. Taken together, the additional auditory information of reach-progression enhances the planning and control of multi-segment reaches and eye–hand coordination at the segment transition.




Similar content being viewed by others
References
Abrahamse EL. Ruitenberg MFL, De Kleine E, Verwey WB (2013) Control of automated behaviour: insights from the discrete sequence production task. Front Hum Neurosci 7:1–16
Adam JJ (1992) The effects of objectives and constraints on motor control strategy in reciprocal aiming movements. J Motor Behav 24:173–185
Adam JJ, Bruggen DPW van der, Bekkering H (1993) The control of discrete and reciprocal target-aiming responses: evidence for the exploitation of mechanics. Hum Mov Sci 12:353–364
Adam JJ, Paas FGWC, Eyssen ICJM, Slingerland H, Bekkering H, Drost M (1995) The control of two-element, reciprocal aiming movements: evidence for chunking. Hum Mov Sci 14:1–11
Adam JJ, Nieuwenstein JH, Huys R, Paas FG, Kingma H, Willems P, Werry M (2000) Control of rapid targeted hand movements: the one-target advantage. J Exp Psychol Hum Percept Perform 26:295–312
Adam JJ, Buetti S, Kerzel D (2012) Coordinated flexibility: how initial gaze position modulates eye-hand coordination and reaching. J Exp Psychol Hum Percept Perform 38:891–901
Adamovich SV, Fluet GG, Mathai A, Qiu Q, Lewis J, Merians AS (2009) Design of a complex virtual reality simulation to train finger motion for persons with hemiparesis: a proof of concept study. J Neuroeng Rehabil 6:28
Alais D, Burr D (2004) No direction-specific bimodal facilitation for audiovisual motion detection. Brain Res Cogn Brain Res 19:185–194
Bekkering H, Adam JJ, van den Aarssen A, Kingma H, Whiting HTA (1995) Interference between saccadic eye and goal-directed hand movements. Exp Brain Res 106:475–484
Bernstein N (1967) The coordination and regulation of movements. Pergamon Press, London
Bieńkiewicz MM, Young WR, Craig CM (2004) Balls to the wall: how acoustic information from a ball in motion guides interceptive movement in people with Parkinson’s disease. Neuroscience 275:508–518
Bowman MC, Johansson RS, Flanagan JR (2009) Eye-hand coordination in a sequential target contact task. Exp Brain Res 195:273–283
Bresciani JP, Dammeier F, Ernst MO (2006) Vision and touch are automatically integrated for the perception of sequences of events. J Vis 6:554–564
Cheng K, Shettleworth SJ, Huttenlocher J, Rieser JJ (2007) Bayesian integration of spatial information. Psychol Bull 133:625–637
Christina RW, Rose DJ (1985) Premotor and motor reaction time as a function of response complexity. Res Q Exerc Sport 56:306–315
Colonius H, Diederich A (2017) Formal models and quantitative measures of multisensory integration: a selective overview. E J Neurosci. https://doi.org/10.1111/ejn.13813
Crawford JD, Medendorp WP, Marotta JJ (2004) Spatial transformations for eye-hand coordination. J Neurophysiol 92:10–19
Debats NB, Heuer H (2018) Optimal integration of actions and their visual effects is based on both online and prior causality evidence. Sci Rep 8:9796
Debats NB, Ernst MO, Heuer H (2017a) Perceptual attraction in tool-use: evidence for a reliability-based weighting mechanism. J Neurophysiol 117:1569–1580
Debats NB, Ernst MO, Heuer H (2017b) Kinematic cross-correlation induces sensory integration across separate objects. E J Neurosci 46:2826–2834
Driver J, Spence C (1998) Attention and the crossmodal construction of space. Trends Cogn Sci 2:254–262
Elliott D (1988) The influence of visual target and limb information on manual aiming. Can J Psychol 42:57–68
Elliott D, Hansen S, Grierson LE, Lyons J, Bennett SJ, Hayes SJ (2010) Goal-directed aiming: two components but multiple processes. Psychol Bull 136:1023–1044
Ernst MO, Banks MS (2002) Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415:429–433
Ernst MO, Bülthoff HH (2004) Merging the senses into a robust percept. Trends Cogn Sci 8:162–169
Fischman MG, Reeve TG (1992) Slower movement times may not necessarily imply on-line programming. J Hum Mov Stud 22:131–144
Fitts PM (1954) The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47:381–391
Flanagan JR, Johansson RS (2003) Action plans used in action observation. Nature 424:769–771
Flanagan JR, Bowman MC, Johansson RS (2006) Control strategies in object manipulation tasks. Curr Opin Neurobiol 16:650–659
Gepshtein S, Burge J, Ernst MO, Banks MS (2005) The combination of vision and touch depends on spatial proximity. J Vis 5:1013–1023
Giard MH, Peronnet F (1999) Auditory-visual integration during multimodal object recognition in humans: a behavioral and electrophysiological study. J Cogn Neurosci 11:473–490
Gielen CCAM, Van den Heuvel PJM, Van Gisbergen JAM (1984) Coordination of fast eye and arm movements in a tracking task. Exp Brain Res 56:156–161
Harrar V, Harris LR (2008) The effect of exposure to asynchronous audio, visual, and tactile stimulus combinations on the perception of simultaneity. Exp Brain Res 186:517–524
Harrar V, Harris LR, Spence C (2017) Multisensory integration is independent of perceived simultaneity. Exp Brain Res 235:763–775
Jeannerod M, Arbib MA, Rizzolatti G, Sakata H (1995) Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci 18:314–320
Johansson RS, Westling G, Bäckström A, Flanagan JR (2001) Eye-hand coordination in object manipulation. J Neurosci 21:6917–6932
Kagerer FA, Contreras-Vidal JL (2009) Adaptation of sound localization induced by rotated visual feedback in reaching movements. Exp Brain Res 193:315–321
Ketcham CJ, Seidler RD, Van Gemmert AWA, Stelmach GE (2002) Age-related kinematic differences as influenced by task difficulty, target size, and movement amplitude. J Gerontol: Psychol Sci 57B:P54–P64
Khan MA, Mottram TM, Adam JJ, Buckolz E (2010) Sequential aiming with two limbs and the one-target advantage. J Mot Behav 42:325–330
Lashley KS (1951) The problem of serial order in behavior. In: Jeffress LA (ed) Cerebral mechanisms in behavior. Wiley, New York, pp 112–136
Marteniuk RG, MacKenzie CL, Jeannerod M, Athenes S, Dugas C (1987) Constraints on human arm movement trajectories. Can J Psychol 41:365–378
Ma-Wyatt A, Stritzke M, Trommershäuser J (2010) Eye-hand coordination while pointing rapidly under risk. Exp Brain Res 203:131–145
Meyer DE, Abrams RA, Kornblum S, Wright CE, Smith JEK (1988) Optimality in human motor performance: ideal control of rapid aimed movements. Psychol Rev 95:340–370
Molholm S, Ritter W, Murray MM, Javitt DC, Schroeder CE, Foxe JJ (2002) Multisensory auditory–visual interactions during early sensory processing in humans: a high-density electrical mapping study. Brain Res Cogn Brain Res 14:115–128
Neggers SFW, Bekkering H (2000) Ocular gaze is anchored to the target of an ongoing pointing movement. J Neurophysiol 83:639–651
Neggers SFW, Bekkering H (2001) Gaze anchoring to a pointing target is present during the entire pointing movement and is driven by a non-visual signal. J Neurophysiol 86:961–970
Paillard J (1982) The contribution of peripheral and central vision to visually guided reaching. In: Ingle D, Goodale M, Mansfield R (eds) Analysis of visual behaviour. MIT Press, Cambridge, pp 367–385
Pelz J, Hayhoe M, Loeber R (2001) The coordination of eye, head, and hand movements in a natural task. Exp Brain Res 139:266–277
Posner MI, Nissen MJ, Klein RM (1976) Visual dominance: an information-processing account of its origins and significance. Psychol Rev 83:157–171
Povel D-J, Collard R (1982) Structural factors in patterned finger tapping. Acta Psychol (Amst) 52:107–123
Rand MK (2014) Segment interdependency and gaze anchoring during manual two–segment sequences. Exp Brain Res 232:2753–2765
Rand MK, Shimansky YP (2013) Two-phase strategy of neural control for planar reaching movements. I. XY coordination variability and its relation to end-point variability. Exp Brain Res 225:55–73
Rand MK, Stelmach GE (2000) Segment interdependency and difficulty in two-stroke sequences. Exp Brain Res 134:228–236
Rand MK, Stelmach GE (2010) Effects of hand termination and accuracy constraint on eye-hand coordination during sequential two-segment movements. Exp Brain Res 207:197–211
Rand MK, Stelmach GE (2011) Adaptation of gaze anchoring through practice in young and older adults. Neurosci Lett 492:47–51
Rand MK, Stelmach GE (2012) Effect of aging on coordinated eye and hand movements with two-segment sequence. Mot Control 16:447–465
Rand MK, Alberts JL, Stelmach GE, Bloedel JR (1997) The influence of movement segment difficulty on movements with two-stroke sequence. Exp Brain Res 115:137–146
Robertson JV, Hoellinger T, Lindberg P, Bensmail D, Hanneton S, Roby-Brami A (2009) Effect of auditory feedback differs according to hemiparesis: a comparative pilot study. J Neuroeng Rehabil 6:45
Rosati G, Oscari F, Spagnol S, Avanzini F, Masiero S (2012) Effect of task-related continuous auditory feedback during learning of tracking motion exercises. J Neuroeng Rehabil 9:79
Rosenbaum DA (1987) Successive approximations to a model of human motor programming. In: Bower G (ed) The psychology of learning and motivation. Academic Press, New York, pp 153–182
Rosenbaum DA (1991) Human motor control. Academic Press, San Diego
Sanders JA, Knill DC (2004) Visual feedback control of hand movements. J Neurosci 24:3223–3234
Schmidt RA, Zelaznik HN, Hawkins B, Frank JS, Quinn JT (1979) Motor output variability: a theory for the accuracy of rapid motor acts. Psychol Rev 86:415–451
Sedda A, Monaco S, Bottini G, Goodale MA (2011) Integration of visual and auditory information for hand actions: preliminary evidence for the contribution of natural sounds to grasping. Exp Brain Res 209:365–374
Shimansky YP (2007) Role of optimization in simple and learning based adaptation and its biologically plausible mechanisms. In: Williams TO (ed) Biological cybernetics research trends. Nova Science Publishers, Hauppauge, pp 95–164
Shimansky YS, Rand MK (2013) Two-phase strategy of controlling motor coordination determined by task performance optimality. Biol Cybern 107:107–129
Short MW, Fischman MG, Wang YT (1996) Cinematographical analysis of movement pathway constraints in rapid target-striking tasks. J Mot Behav 28:157–163
Sidaway B, Sekiya H, Fairweather M (1995) Movement variability as a function accuracy demand in programmed serial aiming responses. J Mot Behav 27:67–76
Sigrist R, Rauter G, Marchal-Crespo L, Riener R, Wolf P (2015) Sonification and haptic feedback in addition to visual feedback enhances complex motor task learning. Exp Brain Res 233:909–925
Smeets JBJ, van den Dobbelsteen JJ, de Grave DDJ, van Beers MJ, Brenner E (2006) Sensory integration does not lead to sensory calibration. Proc Natl Acad Sci 103:18781–18786
Soechting JF (1984) Effect of target size on spatial and temporal characteristics of a pointing movement in man. Exp Brain Res 54:121–132
Spence C, Driver J (1997) On measuring selective attention to an expected sensory modality. Percept Psychophys 59:389–403
Sternberg S, Knoll RL, Turock DL (1990) Hierarchical control in the execution of action sequences: test of two invariance properties. In: Jeannerod M (ed) Attention and performance XIII. Erlbaum, Hillsdale, pp 3–55
Teasdale N, Bard C, Fleury M, Young D, Proteau L (1993) Determining movement onsets from temporal series. J Mot Behav 25:97–106
Todorov E (2004) Optimality principles in sensorimotor control. Nat Neurosci 7:907–915
van Beers RJ, Sittig AC, Denier van der Gon JJ (1999) Integration of proprioceptive and visual position-information: An experimentally supported model. J Neurophysiol 81:1355–1364
van Beers RJ, Wolpert DM, Haggard P (2002) When feeling is more important than seeing in sensorimotor adaptation. Curr Biol 12:834–837
Van der Burg E, Olivers CN, Bronkhorst AW, Theeuwes J (2008) Pip and pop: nonspatial auditory signals improve spatial visual search. J Exp Psychol Hum Percept Perform 34:1053–1065
Verwey WB (1999) Evidence for a multistage model of practice in a sequential movement task. J Exp Psychol Hum Percept Perform 25:1693–1708
Verwey WB, Shea HC, Wright DL (2015) A cognitive framework for explaining serial processing and sequence execution strategies. Psychon Bull Rev 22:54–77
Vindras P, Viviani P (2005) Planning short pointing sequences. Exp Brain Res 160:141–153
Weiss P, Stelmach GE, Hefter H (1997) Programming of a movement sequence in Parkinson’s disease. Brain 120:91–102
Wolpert DM, Flanagan JR (2001) Motor prediction. Curr Biol 11:R729–R732
Woodworth RS (1899) The accuracy of voluntary movement. Psychol Rev 3(Suppl. 2):1–114
Zahariev MA, MacKenzie CL (2007) Grasping at ‘thin air’: multimodal contact cues for reaching and grasping. Exp Brain Res 180:69–84
Zahariev MA, Mackenzie CL (2008) Auditory contact cues improve performance when grasping augmented and virtual objects with a tool. Exp Brain Res 186:619–627
Acknowledgements
This research was supported by the German Research Foundation (DFG), Ra 2183/1–3. The author thanks Anika Beyer, Maleen Greine, and Franziska Schywalski for their support in data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interest.
Appendices
Appendix 1: An assessment of a practice effect due to two different orders of auditory conditions
In the present study, half of the participants used the order A (Fix–Ran–NoS–Ran–Fix–NoS) of auditory conditions, while the other half used the order B (NoS–Fix–Ran–NoS–Ran–Fix). Thus, it is important to ensure that differences among the auditory conditions observed in the present study were not just artifacts of practice that could stem from the different orders of auditory conditions (i.e., order A vs B). Thus, we tested this aspect for all significant results of the sound-type effect and its interaction with other factors. Namely, we applied a 2 (group: order A, order B) × 2 (T1 size: large, small) × 2 (T2 size: large, small) × 3 (sound type: Fix, Ran, NoS) ANOVA with repeated measures, where group was a between-subject factor, in order to see whether the group factor had an interaction with the sound-type factor. The results were as follows.
Regarding the parameters that had a significant sound-type effect (reported in the main text), a significant interaction between group and sound type was found for movement time (F(2,36) = 4.51, p = 0.018) and deceleration time (F(2,36) = 4.14, p = 0.024). Conversely, there was no such an interaction for constant error in the X-position (F(2,36) = 0.14, p = 0.870), peak velocity (F(2,36) = 1.72, ɛ = 0.74, p = 0.203), time to peak velocity (F(2,36) = 2.30, p = 0.115), inter-segment interval (F(2,36) = 0.17, p = 0.846), and handoffset-to-eyeonset dwell time (F(2,36) = 0.92, p = 0.407). Regarding the parameters that had a significant interaction between sound type and T1 size (reported in the main text), there was no significant 3-way interaction among group, sound type, and T1 size in all parameters (hand reaction time: F(2,36) = 0.57, p = 0.566; time to peak velocity: F(2,36) = 1.01, p = 0.374; inter-segment interval: F(2,36) = 1.62, p = 0.211; and handoffset-to-eyeonset dwell time: F(2,36) = 0.26, p = 0.772). These results indicate that the movement time and deceleration time had some practice effect stemming from the different orders (i.e., order A vs B) that affected the results of sound-type effect. In contrast, no other parameters showed any such effect, meaning that differences among auditory conditions were unaffected by the practice effect.
We further assessed the practice effect on the movement time and deceleration time. In the present study, these two parameters showed a similar sound-type main effect (reported in the main text). Namely, the NoS condition had significantly longer duration than the Fix and Ran conditions, while the latter two did not differ from each other (Fig. 3a, d). Thus, the key question here is whether the lengthening of the NoS condition was caused by a pure practice effect or a sound-type effect that remained after taking into account the practice effect. Figure 5 shows mean movement time (plot a) and mean deceleration time (plot d) for three auditory conditions in each order group. Both parameters showed a similar pattern: Namely, both groups increased the durations for the NoS condition compared with the other two conditions. However, the increase was pronounced in the order-B group compared to the order-A group, which led to the above significant group by sound-type interaction.
Supposedly, if there were only a pure practice effect, both parameters for the NoS condition in the order-B group would have increased compared with the other two conditions because the NoS condition preceded the other two, whereas both parameters for the NoS condition in the order-A group would have been decreased because the NoS condition followed the other two. Consequently, the relation between the NoS and the other two conditions would have been reversed between the two groups. However, the results showed that no such reversal occurred in both parameters (Fig. 5a, d) regardless of whether the NoS condition preceded (Group B) or followed (Group A) the other conditions. Therefore, the difference between sound conditions clearly remained despite the practice effect.
Furthermore, when mean movement time and mean deceleration time are plotted against the consecutive order of the 6 sub-conditions for the order-A group (Fig. 5b, e) and order-B group (Fig. 5c, f), it becomes clearer that both parameters for the NoS condition increased compared to the other two conditions (Fig. 5) regardless of whether the NoS condition preceded or followed the other conditions. For example, in the case of the order-A group (Fig. 5b, e), both parameters increased in the NoS condition following the Fix and Ran conditions. Similarly, in the case of the order-B group (Fig. 5c, f), both parameters also increased in the second NoS sub-condition following the first pair of Fix and Ran sub-conditions. If there were only a pure practice effect, the NoS condition would have decreased in both cases. But in reality, both parameters increased in this condition. Therefore, the sound-type effect of the longer NoS condition remained after taking into account the practice effect.
Movement time (a–c) and deceleration time (d–f) of the initial reach are plotted for the order-A group (filled circles) and the order-B group (open circles). Mean values across all participants of each group are plotted for the fixed-timing (Fix), randomized-timing (Ran), and no-sound (NoS) conditions. The order-A group used the consecutive order (Fix–Ran–NoS–Ran–Fix–NoS), whereas the order-B group used another order (NoS–Fix–Ran–NoS–Ran–Fix). In plots a, d, mean values are calculated by pooling the first and second sub-condition within each auditory condition. In plots b, c, e, f, mean values are plotted across the consecutive order for each group. The error bars represent the SE
Appendix 2: An assessment of progressive changes over trials
Since the participants performed each auditory condition as blocks of trials in the present study, there is a possibility that experience from the previous trials about the auditory feedback was stored in memory and used in the planning of subsequent trials. If that were the case, hand movements might improve over trials within the 10-trial blocks. Thus, we tested this aspect for three parameters (hand reaction time, peak velocity, and time to peak velocity) that occurred prior to the delivery of the fixed-timing auditory cue and that showed a significant sound-type effect and/or a significant interaction between sound type and T1 size (reported in the main text).
For each auditory condition of each parameter, we calculated an overall mean of each trial across all participants, two T2 size conditions, and two (first and second) sub-conditions. Next, a change in the means over 10 trials within each auditory condition and each T1 size condition was analyzed by using a linear regression analysis. Peak velocity for the large T1 condition showed a progressive increase over trials in the Fix and Ran conditions (Fig. 6a, b), revealing a significant positive trend across trials (for Fix, correlation coefficient: r = 0.81, p = 0.005, slope = 4.91; for Ran, r = 0.93, p < 0.001, slope = 4.68). Conversely, there was no significant change over trials in the NoS condition (Fig. 6c, r = 0.53, p = 0.112, slope = 2.34). On the other hand, peak velocity for the small T1 condition showed different patterns. There was no progressive change of peak velocity over trials for the Ran and Fix conditions (Fig. 6d, e; Fix: r = 0.40, p = 0.248, slope = -0.82; Ran: r = 0.37, p = 0.296, slope = 1.00), whereas peak velocity significantly decreased over trials for the NoS condition (Fig. 6f, r = 0.73, p = 0.016, slope = − 3.25). Taken together, these results indicate that when auditory feedback is provided, regardless of whether it contains the reach-progress cue, the auditory input contributes to increasing the preplanned speed of initial reach when the reaching accuracy demand is low, but that input contributes to maintaining the speed across trials when the reaching accuracy demand is high.
Contrary to peak velocity, time to peak velocity did not show any significant change over trials in any of the auditory conditions combined with the large T1 (Fix: r = 0.08, p = 0.828, slope = 0.08; Ran: r = 0.20, p = 0.576, slope = − 0.37; NoS: r = 0.20, p = 0.572, slope = − 0.30) or combined with the small T1 (Fix: r = 0.16, p = 0.659, slope = 0.36; Ran: r = 0.38, p = 0.279, slope = 0.55; NoS: r = 0.37, p = 0.297, slope = 0.56). Similarly, hand reaction time did not show significant changes in any of the auditory conditions combined with the large T1 (Fix: r = 0.43, p = 0.219, slope = − 1.37; Ran: r = 0.11, p = 0.763, slope = 0.37; NoS: r = 0.51, p = 0.136, slope = − 1.30) or combined with the small T1 (Fix: r = 0.09, p = 0.800, slope = 0.239; Ran: r = 0.00, p = 0.994, slope = 0.01; NoS: r = 0.01, p = 0.974, slope = − 0.02).
The changes of peak velocity across 10 trials for the fixed-timing (Fix: a, d), randomized-timing (Ran: b, e), and no-sound (NoS: c, f) conditions. Mean values across all participants, two T2 size conditions, and two (first and second) sub-conditions of each auditory condition are plotted against the trial number for the large T1 (a–c) and small T1 (d–f) conditions. Solid lines indicate the linear-equation line
Rights and permissions
About this article
Cite this article
Rand, M.K. Effects of auditory feedback on movements with two-segment sequence and eye–hand coordination. Exp Brain Res 236, 3131–3148 (2018). https://doi.org/10.1007/s00221-018-5366-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5366-4