Abstract
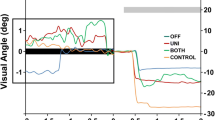
Unilateral deep brain stimulation (DBS) of the subthalamic nucleus (STN) in patients with Parkinson’s disease improves skeletomotor function assessed clinically, and bilateral STN DBS improves motor function to a significantly greater extent. It is unknown whether unilateral STN DBS improves oculomotor function and whether bilateral STN DBS improves it to a greater extent. Further, it has also been shown that bilateral, but not unilateral, STN DBS is associated with some impaired cognitive-motor functions. The current study compared the effect of unilateral and bilateral STN DBS on sensorimotor and cognitive aspects of oculomotor control. Patients performed prosaccade and antisaccade tasks during no stimulation, unilateral stimulation, and bilateral stimulation. There were three sets of findings. First, for the prosaccade task, unilateral STN DBS had no effect on prosaccade latency and it reduced prosaccade gain; bilateral STN DBS reduced prosaccade latency and increased prosaccade gain. Second, for the antisaccade task, neither unilateral nor bilateral stimulation had an effect on antisaccade latency, unilateral STN DBS increased antisaccade gain, and bilateral STN DBS increased antisaccade gain to a greater extent. Third, bilateral STN DBS induced an increase in prosaccade errors in the antisaccade task. These findings suggest that while bilateral STN DBS benefits spatiotemporal aspects of oculomotor control, it may not be as beneficial for more complex cognitive aspects of oculomotor control. Our findings are discussed considering the strategic role the STN plays in modulating information in the basal ganglia oculomotor circuit.




Similar content being viewed by others
Abbreviations
- BOTH:
-
Bilateral stimulation condition
- DBS:
-
Deep brain stimulation
- DLPFC:
-
Dorsolateral prefrontal cortex
- LEFT:
-
Left unilateral stimulation on condition
- MDS-UPDRS:
-
Movement Disorder Society-Unified Parkinson’s Disease Rating Scale
- OFF:
-
Stimulators off condition
- PD:
-
Parkinson’s disease
- RIGHT:
-
Right unilateral stimulation on condition
- SNr:
-
Substantia nigra pars reticulata
- STN:
-
Subthalamic nucleus
- UNI:
-
Unilateral stimulation
References
Alberts JL, Voelcker-Rehage C, Hallahan K, Vitek M, Bamzai R, Vitek JL (2008) Bilateral subthalamic stimulation impairs cognitive-motor performance in Parkinson’s disease patients. Brain 131:3348–3360
Ballanger B, van Eimeren T, Moro E, Lozano AM, Hamani C, Boulinguez P, Pellecchia G, Houle S, Poon YY, Lang AE, Strafella AP (2009) Stimulation of the subthalamic nucleus and impulsivity: release your horses. Ann Neurol 66:817–824
Bastian AJ, Kelly VE, Revilla FJ, Perlmutter JS, Mink JW (2003) Different effects of unilateral versus bilateral subthalamic nucleus stimulation on walking and reaching in Parkinson’s disease. Mov Disord 18:1000–1007
Benarroch EE (2008) Subthalamic nucleus and its connections: anatomic substrate for the network effects of deep brain stimulation. Neurology 70:1991–1995
Benazzouz A, Piallat B, Pollak P, Benabid AL (1995) Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci Lett 189:77–80
Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E (1998) Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci 21:32–38
Campbell MC, Karimi M, Weaver PM, Wu J, Perantie DC, Golchin NA, Tabbal SD, Perlmutter JS, Hershey T (2008) Neural correlates of STN DBS-induced cognitive variability in Parkinson disease. Neuropsychologia 46:3162–3169
Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E (2011) Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol 68:1550–1556
Chiken S, Nambu A (2014) Disrupting neuronal transmission: mechanism of DBS? Front Syst Neurosci 8:33
Condy C, Rivaud-Péchoux S, Ostendorf F, Ploner CJ, Gaymard B (2004) Neural substrate of antisaccades: role of subcortical structures. Neurology 63:1571–1578
Everling S, Johnston K (2013) Control of the superior colliculus by the lateral prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 368:20130068
Fawcett AP, Dostrovsky JO, Lozano AM, Hutchison WD (2005) Eye movement-related responses of neurons in human subthalamic nucleus. Exp Brain Res 162:357–365
Fawcett AP, González EG, Moro E, Steinbach MJ, Lozano AM, Hutchison WD (2010) Subthalamic nucleus deep brain stimulation improves saccades in Parkinson’s disease. Neuromodulation 13:17–25
Folstein MF, Folstein SE, McHugh PR (1975) ”Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM (2004) The subthalamic nucleus in the context of movement disorders. Brain 127:4–20
Harris MS, Reilly JL, Keshavan MS, Sweeney JA (2006) Longitudinal studies of antisaccades in antipsychotic-naive first-episode schizophrenia. Psychol Med 36:485–494
Hedeker D, Gibbons RD (2006) Longitudinal data analysis. John Wiley & Sons, New York
Hikosaka O, Takikawa Y, Kawagoe R (2000) Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80:953–978
Hood AJ, Amador SC, Cain AE, Briand KA, Al-Refai AH, Schiess MC, Sereno AB (2007) Levodopa slows prosaccades and improves antisaccades: an eye movement study in Parkinson’s disease. J Neurol Neurosurg Psychiatry 78:565–570
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Jaffard M, Longcamp M, Velay JL, Anton JL, Roth M, Nazarian B, Boulinguez P (2008) Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage 42:1196–1206
Jiang H, Stein BE, McHaffie JG (2003) Opposing basal ganglia processes shape midbrain visuomotor activity bilaterally. Nature 423:982–986
Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P (2003) Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 349:1925–1934
Kumar R, Lozano AM, Sime E, Halket E, Lang AE (1999) Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology 53:561–566
Limousin P, Greene J, Pollak P, Rothwell J, Benabid AL, Frackowiak R (1997) Changes in cerebral activity pattern due to subthalamic nucleus or internal pallidum stimulation in Parkinson’s disease. Ann Neurol 42:283–291
Magill PJ, Bolam JP, Bevan MD (2001) Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus–globus pallidus network. Neuroscience 106:313–330
Matsumura M, Kojima J, Gardiner TW, Hikosaka O (1992) Visual and oculomotor functions of monkey subthalamic nucleus. J Neurophysiol 67:1615–1632
Maurice N, Thierry AM, Glowinski J, Deniau JM (2003) Spontaneous and evoked activity of substantia nigra pars reticulata neurons during high-frequency stimulation of the subthalamic nucleus. J Neurosci 23:9929–9936
McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL (2004) Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 115:1239–1248
Monakow KH, Akert K, Künzle H (1978) Projections of the precentral motor cortex and other cortical areas of the frontal lobe to the subthalamic nucleus in the monkey. Exp Brain Res 33:395–403
Munoz DP, Broughton JR, Goldring JE, Armstrong IT (1998) Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res 121:391–400
Nambu A (2011) Somatotopic organization of the primate Basal Ganglia. Front Neuroanat 5:26
Nambu A, Tokuno H, Takada M (2002) Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect’ pathway. Neurosci Res 43:111–117
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Pierrot-Deseilligny C, Müri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S (2003) Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain 126:1460–1473
Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, Agid Y, DeLong MR, Obeso JA (2010) Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci 11:760–772
Rivaud-Péchoux S, Vermersch AI, Gaymard B, Ploner CJ, Bejjani BP, Damier P, Demeret S, Agid Y, Pierrot-Deseilligny C (2000) Improvement of memory guided saccades in parkinsonian patients by high frequency subthalamic nucleus stimulation. J Neurol Neurosurg Psychiatry 68:381–384
Sauleau P, Pollak P, Krack P, Courjon JH, Vighetto A, Benabid AL, Pélisson D, Tilikete C (2008) Subthalamic stimulation improves orienting gaze movements in Parkinson’s disease. Clin Neurophysiol 119:1857–1863
Shapiro MB, Vaillancourt DE, Sturman MM, Metman LV, Bakay RA, Corcos DM (2007) Effects of STN DBS on rigidity in Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng 15:173–181
Sturman MM, Vaillancourt DE, Metman LV, Bakay RA, Corcos DM (2004) Effects of subthalamic nucleus stimulation and medication on resting and postural tremor in Parkinson’s disease. Brain 127:2131–2143
Swann N, Poizner H, Houser M, Gould S, Greenhouse I, Cai W, Strunk J, George J, Aron AR (2011) Deep brain stimulation of the subthalamic nucleus alters the cortical profile of response inhibition in the beta frequency band: a scalp EEG study in Parkinson’s disease. J Neurosci 31:5721–5729
Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR (1996) Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. J Neurophysiol 75:454–468
Tabbal SD, Ushe M, Mink JW, Revilla FJ, Wernle AR, Hong M, Karimi M, Perlmutter JS (2008) Unilateral subthalamic nucleus stimulation has a measurable ipsilateral effect on rigidity and bradykinesia in Parkinson disease. Exp Neurol 211:234–242
Temperli P, Ghika J, Villemure JG, Burkhard PR, Bogousslavsky J, Vingerhoets FJ (2003) How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 60:78–81
Thobois S, Dominey P, Fraix V, Mertens P, Guenot M, Zimmer L, Pollak P, Benabid AL, Broussolle E (2002) Effects of subthalamic nucleus stimulation on actual and imagined movement in Parkinson’s disease: a PET study. J Neurol 249:1689–1698
Toleikis JR, Metman LV, Pilitsis JG, Barborica A, Toleikis SC, Bakay RA (2012) Effect of intraoperative subthalamic nucleus DBS on human single-unit activity in the ipsilateral and contralateral subthalamic nucleus. J Neurosurg 116:1134–1143
Torres EB, Heilman KM, Poizner H (2011) Impaired endogenously evoked automated reaching in Parkinson’s disease. J Neurosci 31:17848–17863
Vaillancourt DE, Prodoehl J, Verhagen Metman L, Bakay RA, Corcos DM (2004) Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain 127:491–504
Welter ML, Houeto JL, Bonnet AM, Bejjani PB, Mesnage V, Dormont D, Navarro S, Cornu P, Agid Y, Pidoux B (2004) Effects of high-frequency stimulation on subthalamic neuronal activity in parkinsonian patients. Arch Neurol 61:89–96
Williams AE, Arzola GM, Strutt AM, Simpson R, Jankovic J, York MK (2011) Cognitive outcome and reliable change indices two years following bilateral subthalamic nucleus deep brain stimulation. Parkinsonism Relat Disord 17:321–327
York MK, Dulay M, Macias A, Levin HS, Grossman R, Simpson R, Jankovic J (2008) Cognitive declines following bilateral subthalamic nucleus deep brain stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry 79:789–795
Yugeta A, Terao Y, Fukuda H, Hikosaka O, Yokochi F, Okiyama R, Taniguchi M, Takahashi H, Hamada I, Hanajima R, Ugawa Y (2010) Effects of STN stimulation on the initiation and inhibition of saccade in Parkinson disease. Neurology 74:743–748
Acknowledgements
This study was supported by NIH (R56NS040902). The sponsors were not involved in the design, conduct, collection, management, analysis, and/or interpretation of the study results and preparation, review, or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of NIH.
Funding
LCG has no financial disclosures. FJD received grant support from NIH. JAS received grant support from NIH and has consulted to Takeda Pharmaceuticals. DEV received grant support from the NIH, Tyler’s Hope Foundation, Bachmann Straus Foundation, and is Co-Founder and Manager of Neuroimaging Solutions. HP received grant support from NIH, NSF, and ONR. LVM received grant support from NIH, Michael J. Fox, PDF, and contracts for clinical trials from Boston Scientific, Medtronic, Adamas, Osmotica, USWorldMeds. DMC received grant support from NIH and Michael J. Fox, and receives lecture, honoraria, and reviewer fees from NIH.
Author contributions
LCG was responsible for study concept and design, acquisition of data, analysis and interpretation of the data, statistical analysis, drafting the manuscript, and administrative, technical, or material support. FJD was responsible for study concept and design, obtaining funding, acquisition of data, analysis and interpretation of the data, statistical analysis, drafting the manuscript, and administrative, technical, or material support. JAS was responsible for study concept and design, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and administrative, technical, or material support. DEV and HP were responsible for study concept and design, obtaining funding, interpretation of the data, critical revision of the manuscript for important intellectual content, and administrative, technical, or material support. LVM was responsible for patient recruitment, study concept and design, obtaining funding, study supervision, interpretation of the data, critical revision of the manuscript for important intellectual content, and administrative, technical, or material support. DMC was responsible for study concept and design, obtaining funding, interpretation of the data, critical revision of the manuscript for important intellectual content, study supervision, and administrative, technical, or material support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goelz, L.C., David, F.J., Sweeney, J.A. et al. The effects of unilateral versus bilateral subthalamic nucleus deep brain stimulation on prosaccades and antisaccades in Parkinson’s disease. Exp Brain Res 235, 615–626 (2017). https://doi.org/10.1007/s00221-016-4830-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4830-2