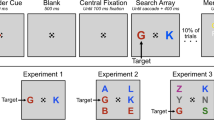
Abstract
Foreknowledge about upcoming events may be exploited to optimize behavioural responses. In a previous work, using an eye movement paradigm, we showed that different types of partial foreknowledge have different effects on saccadic efficiency. In the current study, we investigated the neural circuitry involved in processing of partial foreknowledge using functional magnetic resonance imaging. Fourteen subjects performed a mixed antisaccade, prosaccade paradigm with blocks of no foreknowledge, complete foreknowledge or partial foreknowledge about stimulus location, response direction or task. We found that saccadic foreknowledge is processed primarily within the well-known oculomotor network for saccades and antisaccades. Moreover, we found a consistent decrease in BOLD activity in the primary and secondary visual cortex in all foreknowledge conditions compared to the no-foreknowledge conditions. Furthermore we found that the different types of partial foreknowledge are processed in distinct brain areas: response foreknowledge is processed in the frontal eye field, while stimulus foreknowledge is processed in the frontal and parietal eye field. Task foreknowledge, however, revealed no positive BOLD correlate. Our results show different patterns of engagement in the saccade-related neural network depending upon precisely what type of information is known ahead.






Similar content being viewed by others
References
Abegg M, Manoach DS, Barton JJ (2011) Knowing the future: partial foreknowledge effects on the programming of prosaccades and antisaccades. Vision Res 51:215–221
Barton JJ, Greenzang C, Hefter R, Edelman J, Manoach DS (2006a) Switching, plasticity, and prediction in a saccadic task-switch paradigm. Exp Brain Res 168:76–87
Barton JJ, Kuzin A, Polli F, Manoach DS (2006b) The use of working memory for task prediction: what benefits accrue from different types of foreknowledge? Neuroscience 139:385–392
Buchel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ (1998) The functional anatomy of attention to visual motion. A functional MRI study. Brain J Neurol 121(Pt 7):1281–1294
Bushnell MC, Goldberg ME, Robinson DL (1981) Behavioral enhancement of visual responses in monkey cerebral cortex. I. Modulation in posterior parietal cortex related to selective visual attention. J Neurophysiol 46:755–772
Connolly JD, Goodale MA, Menon RS, Munoz DP (2002) Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci 5:1345–1352
Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL (1998) A common network of functional areas for attention and eye movements. Neuron 21:761–773
Curtis CE, Connolly JD (2008) Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol 99:133–145
Domagalik A, Beldzik E, Fafrowicz M, Oginska H, Marek T (2012) Neural networks related to pro-saccades and anti-saccades revealed by independent component analysis. NeuroImage 62:1325–1333
Fahoum F, Lopes R, Pittau F, Dubeau F, Gotman J (2012) Widespread epileptic networks in focal epilepsies-EEG-fMRI study. Epilepsia 53(9):1618–1627
Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995) Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 33:636–647
Gagnon D, O’Driscoll GA, Petrides M, Pike GB (2002) The effect of spatial and temporal information on saccades and neural activity in oculomotor structures. Brain J Neurol 125:123–139
Grosbras MH, Laird AR, Paus T (2005) Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Mapp 25:140–154
Kobayashi E, Grova C, Tyvaert L, Dubeau F, Gotman J (2009) Structures involved at the time of temporal lobe spikes revealed by interindividual group analysis of EEG/fMRI data. Epilepsia 50:2549–2556
Li CSR, Mazzoni P, Andersen RA (1999) Effect of reversible inactivation of macaque lateral intraparietal area on visual and memory saccades. J Neurophysiol 81:1827–1838
Luck SJ, Chelazzi L, Hillyard SA, Desimone R (1997) Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 77:24–42
Martinez-Trujillo JC, Medendorp WP, Wang H, Crawford JD (2004) Frames of reference for eye-head gaze commands in primate supplementary eye fields. Neuron 44(6):1057–1066
McDowell JE, Dyckman KA, Austin BP, Clementz BA (2008) Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn 68:255–270
Milea D, Lobel E, Lehericy S, Leboucher P, Pochon JB, Pierrot-Deseilligny C, Berthoz A (2007) Prefrontal cortex is involved in internal decision of forthcoming saccades. NeuroReport 18:1221–1224
Moschner C, Zangemeister WH (1993) Preview control of gaze saccades: efficacy of prediction modulates eye–head interaction during human gaze saccades. Neurol Res 15:417–432
Motter BC (1993) Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol 70:909–919
Pare M, Munoz DP (1996) Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol 76:3666–3681
Perry RJ, Zeki S (2000) The neurology of saccades and covert shifts in spatial attention: an event-related fMRI study. Brain J Neurol 123(Pt 11):2273–2288
Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Rivaud-Pechoux S (2003) Cortical control of ocular saccades in humans: a model for motricity. Prog Brain Res 142:3–17
Pierrot-Deseilligny C, Milea D, Muri RM (2004) Eye movement control by the cerebral cortex. Curr Opin Neurol 17:17–25
Ptak R, Muri RM (2013) The parietal cortex and saccade planning: lessons from human lesion studies. Front Hum Neurosci 7:254
Schiller PH, Haushofer J, Kendall G (2004) How do target predictability and precueing affect the production of express saccades in monkeys? Eur J Neurosci 19:1963–1968
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Georg Thieme, Stuttgart; New York, p 122
Vernet M, Quentin R, Chanes L, Mitsumasu A, Valero-Cabre A (2014) Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Front Integr Neurosci 8:66
Wiest R, Estermann L, Scheidegger O, Rummel C, Jann K, Seeck M, Schindler K, Hauf M (2013) Widespread grey matter changes and hemodynamic correlates to interictal epileptiform discharges in pharmacoresistant mesial temporal epilepsy. J Neurol 260:1601–1610
Yarbus A (1967) Eye movements and vision. Plenum Press, New York, p 129
Acknowledgments
MA was supported by the Swiss National Science Foundation. JB was supported by a Canada Research Chair and the Marianne Koerner Chair in Brain Diseases. This work was supported by CIHR grant MOP-81270.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicting relationship exists for any author.
Rights and permissions
About this article
Cite this article
Bär, S., Hauf, M., Barton, J. .S. et al. The neural network of saccadic foreknowledge. Exp Brain Res 234, 409–418 (2016). https://doi.org/10.1007/s00221-015-4468-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4468-5