Abstract
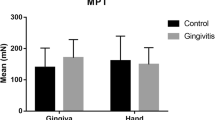
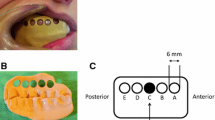
We aimed to evaluate the effect of painful tooth stimulation on gingival somatosensory sensitivity of healthy volunteers in a randomized, controlled design. Thirteen healthy volunteers (six women, seven men; 28.4 ± 5.0 years) were included for two experimental sessions of electrical tooth stimulation: painful tooth stimulation and tooth stimulation below the sensory threshold (control). Eight of the human subjects participated in a third session without tooth stimulation. In all sessions, the somatosensory sensitivity of the gingiva adjacent to the stimulated tooth was evaluated with a standardized battery of quantitative sensory tests (QST) before, immediately after and 30 min after tooth stimulation. Painful tooth stimulation evoked significant decreases in warmth and heat pain thresholds (P < 0.001) as well as pressure pain thresholds (increased sensitivity) (P = 0.024) and increases in mechanical detection thresholds (decreased sensitivity) (P < 0.050). Similar thermal threshold changes (P < 0.019) but no mechanical changes were found after tooth stimulation below the sensory threshold (P > 0.086). No QST changes were detected in the session without tooth stimulation (P > 0.060). In conclusion, modest increased gingival sensitivity to warmth, painful heat and pressure stimuli as well as desensitization to non-painful mechanical stimulation were demonstrated after tooth stimulation. This suggests involvement of competing heterotopic facilitatory and inhibitory mechanisms. Furthermore, stimulation below the sensory threshold induced similar thermal sensitization suggesting the possibility of activation of axon-reflex-like mechanisms even at intensities below the perception threshold. These findings may have implications for interpretation of somatosensory results in patients with chronic intraoral pain.




Similar content being viewed by others
References
Avellan NL, Sorsa T, Tervahartiala T, Mantyla P, Forster C, Kemppainen P (2005) Painful tooth stimulation elevates matrix metalloproteinase-8 levels locally in human gingival crevicular fluid. J Dent Res 84:335–339
Avellan NL, Sorsa T, Tervahartiala T, Forster C, Kemppainen P (2008) Experimental tooth pain elevates substance P and matrix metalloproteinase-8 levels in human gingival crevice fluid. Acta Odontol Scand 66:18–22
Baad-Hansen L (2008) Atypical odontalgia—pathophysiology and clinical management. J Oral Rehabil 35:1–11
Baad-Hansen L, Leijon G, Svensson P, List T (2008) Comparison of clinical findings and psychosocial factors in patients with atypical odontalgia and temporomandibular disorders. J Orofac Pain 22:7–14
Baad-Hansen L, Pigg M, Ivanovic SE, Faris H, List T, Drangsholt M, Svensson P (2013a) Chairside intraoral qualitative somatosensory testing: reliability and comparison between patients with atypical odontalgia and healthy controls. J Orofac Pain 27:165–170
Baad-Hansen L, Pigg M, Ivanovic SE, Faris H, List T, Drangsholt M, Svensson P (2013b) Intraoral somatosensory abnormalities in patients with atypical odontalgia-a controlled multicenter quantitative sensory testing study. Pain 154:1287–1294
Baad-Hansen L, Pigg M, Yang G, List T, Svensson P, Drangsholt M (2014) Reliability of intra-oral quantitative sensory testing (QST) in patients with atypical odontalgia and healthy controls—a multicentre study. J Oral Rehabil. doi:10.1111/joor.12245
Benoliel R, Zadik Y, Eliav E, Sharav Y (2012) Peripheral painful traumatic trigeminal neuropathy: clinical features in 91 cases and proposal of novel diagnostic criteria. J Orofac Pain 26:49–58
Brown AC, Beeler WJ, Kloka AC, Fields RW (1985) Spatial summation of pre-pain and pain in human teeth. Pain 21:1–16
Chiang CY, Park SJ, Kwan CL, Hu JW, Sessle BJ (1998) NMDA receptor mechanisms contribute to neuroplasticity induced in caudalis nociceptive neurons by tooth pulp stimulation. J Neurophysiol 80:2621–2631
De Col R, Maihöfner C (2008) Centrally mediated sensory decline induced by differential C-fiber stimulation. Pain 138:556–564
Forssell H, Jääskeläinen S, List T, Svensson P, Baad-Hansen L (2014) An update on pathophysiological mechanisms related to idiopathic orofacial pain conditions with implications for management. J Oral Rehabil. doi:10.1111/joor.12256
Gracely RH, Dubner R, McGrath PA (1979) Narcotic analgesia: fentanyl reduces the intensity but not the unpleasantness of painful tooth pulp sensations. Science 203:1261–1263
Gracely RH, Dubner R, McGrath PA (1982) Fentanyl reduces the intensity of painful tooth pulp sensations: controlling for detection of active drugs. Anesth Analg 61:751–755
Holzer P (1988) Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 24:739–768
Hu JW, Sessle BJ (1989) Effects of tooth pulp deafferentation on nociceptive and nonnociceptive neurons of the feline trigeminal subnucleus caudalis (medullary dorsal horn). J Neurophysiol 61:1197–1206
Hu JW, Dostrovsky JO, Lenz YE, Ball GJ, Sessle BJ (1986) Tooth pulp deafferentation is associated with functional alterations in the properties of neurons in the trigeminal spinal tract nucleus. J Neurophysiol 56:1650–1668
Hu JW, Sharav Y, Sessle BJ (1990) Effects of one- or two-stage deafferentation of mandibular and maxillary tooth pulps on the functional properties of trigeminal brainstem neurons. Brain Res 516:271–279
Hu JW, Shohara E, Sessle BJ (1992) Patterns and plasticity of dental afferent inputs to trigeminal (V) brainstem neurons in kittens. Proc Finn Dent Soc 88(Suppl 1):563–569
Janig W, Zimmermann M (1971) Presynaptic depolarization of myelinated afferent fibres evoked by stimulation of cutaneous C fibres. J Physiol 214:29–50
Juhl GI, Jensen TS, Nørholt SE, Svensson P (2008) Central sensitization phenomena after third molar surgery: a quantitative sensory testing study. Eur J Pain 12:116–127
Kemppainen P, Leppanen H, Jyvasjarvi E, Pertovaara A (1994) Blood flow increase in the orofacial area of humans induced by painful stimulation. Brain Res Bull 33:655–662
Kemppainen P, Forster C, Handwerker HO (2001) The importance of stimulus site and intensity in differences of pain-induced vascular reflexes in human orofacial regions. Pain 91:331–338
Kemppainen P, Avellan NL, Handwerker HO, Forster C (2003) Differences between tooth stimulation and capsaicin-induced neurogenic vasodilatation in human gingiva. J Dent Res 82:303–307
List T, Leijon G, Svensson P (2008) Somatosensory abnormalities in atypical odontalgia: a case-control study. Pain 139:333–341
Magerl W, Treede RD (2004) Secondary tactile hypoesthesia: a novel type of pain-induced somatosensory plasticity in human subjects. Neurosci Lett 361:136–139
Magerl W, Szolcsanyi J, Westerman RA, Handwerker HO (1987) Laser Doppler measurements of skin vasodilation elicited by percutaneous electrical stimulation of nociceptors in humans. Neurosci Lett 82:349–354
Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihöfner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede RD (2010) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 150:439–450
Matthews B, Baxter J, Watts S (1976) Sensory and reflex responses to tooth pulp stimulation in man. Brain Res 113:83–94
McGrath PA, Sharav Y, Dubner R, Gracely RH (1981) Masseter inhibitory periods and sensations evoked by electrical tooth pulp stimulation. Pain 10:1–17
McGrath PA, Gracely RH, Dubner R, Heft MW (1983) Non-pain and pain sensations evoked by tooth pulp stimulation. Pain 15:377–388
Melis M, Secci S (2007) Diagnosis and treatment of atypical odontalgia: a review of the literature and two case reports. J Contemp Dent Pract 8:81–89
Melis M, Lobo SL, Ceneviz C, Zawawi K, Al Badawi E, Maloney G, Mehta N (2003) Atypical odontalgia: a review of the literature. Headache 43:1060–1074
Motohashi K, Umino M, Fujii Y (2002) An experimental system for a heterotopic pain stimulation study in humans. Brain Res Brain Res Protoc 10:31–40
Nixdorf DR, Drangsholt MT, Ettlin DA, Gaul C, De Leeuw R, Svensson P, Zakrzewska JM, De Laat A, Ceusters W (2012) Classifying orofacial pains: a new proposal of taxonomy based on ontology. J Oral Rehabil 39:161–169
Park SJ, Chiang CY, Hu JW, Sessle BJ (2001) Neuroplasticity induced by tooth pulp stimulation in trigeminal subnucleus oralis involves NMDA receptor mechanisms. J Neurophysiol 85:1836–1846
Pigg M, Baad-Hansen L, Svensson P, Drangsholt M, List T (2010) Reliability of intraoral quantitative sensory testing (QST). Pain 148:220–226
Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD (2006) Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain 10:77–88
Sessle BJ (2000) Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med 11:57–91
Sharav Y, McGrath PA, Dubner R (1982) Masseter inhibitory periods and sensations evoked by electrical tooth pulp stimulation in patients with oral-facial pain and mandibular dysfunction. Arch Oral Biol 27:305–310
Svensson P, Baad-Hansen L, Pigg M, List T, Eliav E, Ettlin D, Michelotti A, Tsukiyama Y, Matsuka Y, Jääskeläinen SK, Essick G, Greenspan JD, Drangsholt M (2011) Guidelines and recommendations for assessment of somatosensory function in oro-facial pain conditions—a taskforce report. J Oral Rehabil 38:366–394
Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J (2008) Neuropathic pain redefinition and a grading system for clinical and research purposes. Neurology 70:1630–1635
Virtanen ASJ, Huopaniemi T, Närhi M, Pertovaara A, Wallgren K (1987) The effect of temporal parameters on subjective sensations evoked by electrical tooth stimulation. Pain 30:361–371
Woda A, Pionchon P (1999) A unified concept of idiopathic orofacial pain: clinical features. J Orofac Pain 13:172–184
Woda A, Pionchon P (2000) A unified concept of idiopathic orofacial pain: pathophysiologic features. J Orofac Pain 14:196–212
Woolf CJ (2004) Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 140:441–451
Zimmermann M (1968) Dorsal root potentials after C-fiber stimulation. Science 160:896–898
Acknowledgments
This study was financially supported by the Aarhus University Research Foundation, Beijing Stomatological Hospital research funding 13-09-06 and Capital Medical University research funding 14-JL-77.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baad-Hansen, L., Lu, S., Kemppainen, P. et al. Differential changes in gingival somatosensory sensitivity after painful electrical tooth stimulation. Exp Brain Res 233, 1109–1118 (2015). https://doi.org/10.1007/s00221-014-4186-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-014-4186-4