Abstract
In this work a simple and rapid automated analytical method for the determination of sulfur dioxide in wines by employing a sequential automatic analyser was validated. The methodology is based on the reaction of sulfur dioxide with 5,5′-dithiobis-(2-nitrobenzoic acid) in an alkaline medium followed by photometric detection. The linearity of the calibration curve was excellent (R2 = 1.000) and the LOQ (2.00 mg L−1) was well suited for the monitoring of regulatory limits. The accuracy and precision were evaluated with reference materials, providing highly successful results (RSD% < 3.8 for precision and R2 = 0.99 of correlation between the obtained and declared values). The method was applied to the analysis of commercial samples of wines ensuring compliance with legislation. The results found were compared to those obtained with the spectrophotometric method recommended for beers, implemented in a flow system, showing that there are no statistical differences between them (α ≤ 0.05). When compared with conventional methods, the proposed methodology showed to be fast, with minimum handling of the sample and small sample and reagents volume requirement, which together with a minimum maintenance, involves a considerable reduction in laboratory costs.
Similar content being viewed by others
Introduction
Sulfur dioxide is an additive commonly used in the food industry because its excellent preservative properties to inhibit bacterial growth, to control enzymatic and non-enzymatic browning reactions, and to prevent oxidation during elaboration and storage [1]. It is also found naturally in foods due to fermentative processes by yeasts and because of metabolism of aminoacids containing sulfur groups [2]. In the European Union the use of sulfur dioxide and several inorganic sulfites (E220-E228) as food additives is authorized according to the regulation CE no 1333/2008 [3]. Regarding the wine industry, sulfiting agents are the most employed additives since without this additive is not possible to guarantee the wine quality [4]. The admitted sulfiting compounds in the winemaking by the European Union and the International Organization of Vine and Wine (OIV) are liquid sulfur dioxide (SO2, E220), potasium metabisulfite (K2S2O5, E224) and potasium hydrogen sulfite (KHSO3, E228) [5, 6].
Sulfur dioxide may be present in foods in two forms: “free SO2” and “bound SO2”. The free SO2 is the effective form and includes inorganic forms depending on the sample pH: SO2 at pH < 3, HSO3− at pH values in the range 3–7, SO32− at pH > 7. Since the pH of wine usually ranges from 3.0–3.6, bisulfite is the predominant form. The bound SO2 comprises covalents adducts formed with different organic compounds containing carbonyl groups, mainly sugars. Free and bound SO2 are in a reversible equilibrium and the sum of both forms corresponds to total sulfur dioxide [1].
Sulfur dioxide is safe for the majority of people but in the 1980s some studies reported that it presents toxicity in sensitive individuals since high concentrations can cause noxious effects such as allergic reactions, dermatological problems and respiratory symptoms, especially in asthmatic individuals [2]. As a consequence the Joint FAO/WHO fixed the Acceptable Daily Intake of 0.7 mg kg−1 body weight per day [7]. Therefore the use of SO2 must be rigorously controlled and in the European Union and United States, the legislation establishes that if the concentration of total sulfur dioxide is higher than 10 mg L−1, it must be declared on the label of the product [8, 9].
The use of sulfur dioxide is one of the issues of contemporary oenology since the control of sulfur dioxide content is crucial during winemaking not only to assess the wine quality and because of health hazards but also because high concentrations of SO2 affect to colour stability, taste and flavour, spicy odour. As a consequence, the total sulfur dioxide content is one of the parameters which must be analysed in wines as established in Legislation [10]. The European Union according to regulation EU no 2019/934 [5] has set the maximum permitted level of total sulfur dioxide which depends on the type of wine as well as the content of sugar in the wine: 150 mg L−1 for red wines and 200 mg L−1 for white and rosés wines (containing at the most 5 mg L−1 sugar); 200 mg L−1 for red wines and 250 mg L−1 for white and rosés wines (containing more than 5 mg L−1 sugar); 300–400 mg L−1 for specific sweet wines.
Due to the great relevance of monitoring the sulfur dioxide content in wines, numerous procedures have been described for its determination. The two commonly accepted methods by authorities and the wine industry for evaluating sulfur dioxide in wines are the optimised Monier Williams [11, 12] and the Ripper [13] methods. The Monier Williams method consists of a distillation under acidic conditions followed by an oxidation with hydrogen peroxide and subsequent titration of the sulfuric acid formed with sodium hydroxide. Although this method has the advantage of low cost, the procedure is laborious, time consuming and it is limited by interferences due to the co-distillation of volatile acidic compounds. The Ripper method, which is the most widely used method in wineries and cellars, is based on the sulfur dioxide titration with iodine using starch as an indicator. This method is relatively rapid, cheap and simple; however, the Ripper method suffers from interferences of polyphenols, ascorbic acid and other reductants and it presents difficulties to observe a change of colour at the end point of titration, especially in red wines.
To overcome these limitations other techniques have been developed, being UV–Vis spectrophotometry the most widely used technique for the determination of sulfur dioxide in alcoholic beverages. These methods are based on the reaction of sulfur dioxide with organic reagents, such as p-rosaniline [14], malachite green [15] and 5,5′-dithiobis-(2-nitrobenzoic acid) [16]. Chromatographic techniques such as gas chromatography with an electron capture detector [17] and ion chromatography with a conductivity detector [18] and electrochemical techniques such as amperometry [19] and voltammetry [20] have been also employed.
Automated methodologies based on flow analysis have been also utilized. This methodology is based on the diffusion of sulfur dioxide through a gas-permeable membrane from the acidified wine sample (pH < 1) to an acceptor stream. The most usual detection technique is colorimetric having a reagent on the acceptor channel such as p-rosaniline or malachite green [15]. Other used detection techniques are potentiometry [21] or voltammetry [22].
Food standard authorities are increasingly demand methods for routine analysis which require the implementation of instrumental techniques that deliver information quickly, inexpensively and with high accuracy. In this sense, automatic discrete analysers are rapid, automatic and flexible, thus their implementation in the wine industry for routine quality control is of great interest. In 2018, the OIV considering the interest of the experts of SubCommsion of Analytical methods in the use of automated methods, added the enzymatic determination of D-glucose and D-fructose and three organic acids (acetic, lactic and malic) by using an automatic sequential analyser to the Compendium of International Methods of Analysis of Wines and Musts [23].
Therefore, the main aim of this work was the validation of an automated photometric method employing a sequential analyser for the determination of sulfur dioxide in wines. The method is based on the reaction between sulfur dioxide and 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) in basic conditions. The method was applied to 37 commercial samples of wine from Galicia (Northwestern Spain), where the industry of wine is very important. As mentioned above, since the recommended methods present positive interferences, the obtained results were compared with those achieved by using a segmented flow analyser employing the widely used p-rosaniline-formaldehyde method, which is recommended for determining sulfur dioxide in beers [24].
Materials and methods
Samples
For this study, 37 commercial samples of wine produced in different vine-growing areas of Galicia (Northwestern Spain) were analysed. All the wine bottle samples were supplied directly from the cellars and stored out of light exposure prior to the analysis. Due to sulfur dioxide being volatile and can be rapidly oxidized, bottles were opened just before analysis. Precision and accuracy were evaluated using reference materials obtained by participating in an interlaboratory comparison test organised by the Interprofessional Bureau of Analytical Studies (BIPEA, Paris, France). All wines were analysed without any sample pretreatment.
Chemicals and reagents
Sodium metabisulfite (99%) and p-rosaniline chloride were obtained from Sigma-Aldrich (Inc. St. Louis, MO, USA). Hydrochloric acid (37%), sulfuric acid (96%) and formaldehyde (37–38%) were supplied by Panreac (Barcelona, Spain). A stock standard solution of 1500 mg L−1 of sodium metabisulfite (1000 mg L−1 as SO2) was prepared using Milli-Q water obtained from a purification system from Millipore (Billerica, MA, USA), and stored at 4 °C in the dark. A control solution of sulfite of 80 mg L−1 and total sulfite assay kit (DTNB) constituted by buffer at pH 8.2, DTNB at pH 8.2 and standard of sulfite (150 mg L−1 as SO2) were purchased from Biosystems (Barcelona, Spain).
Discrete sequential analyser
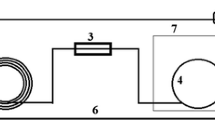
Analyses were carried out using an automatic analyser from Biosystems (model Y15) equipped with a UV detector and software to control operation and process data. The procedure is based on the reaction of SO2 with 5,5′-dithiobis (2-nitrobenzoic acid) in an alkaline medium at pH 8.2 to generate a yellow thiol, 5-mercapto-2-nitrobenzoate, whose absorbance is measured at 405 nm. The reaction is carried out in an alkaline medium to release all the bound sulfur dioxide from the wine sample and convert the hydrogen sulfite to sulfite. For each analysis, 7 μL of sample and 300 μL of reagent (240 μL of buffer and 60 μL solution of DTNB at pH 8.2) were used. The reaction was carried out at room temperature for 7 min. The robotic sampling arm dispenses precise quantities of sample and reagents into a reaction cuvette placed in a reaction rotor. Once the reaction is completed, the cuvette is moved to the photometer and the resultant absorbance is measured. As an illustration, Fig. 1 schematizes the analytical procedure.
Segmented flow analyser
Analyses were carried out using a segmented flow analyser from Astoria-Pacific (Clackamas, OR, USA). The system consisted of a sampling device, a peristaltic pump, a heating unit, a gas diffusion module with two chambers separated by a gas permeable membrane, a reaction cartridge and a density optical detector. Data were processed using FASPac 2 software (Astoria-Pacific). The SO2 determination is based on the reaction of SO2 with p-rosaniline in the presence of formaldehyde at pH 0.9 to form a sulfur dioxide/formaldehyde/p-rosaniline complex, whose optical density is monitored at 560 nm.
The sample is aspired by the sampling device and pumped and mixed with the carrier solution (hydrochloric acid, pH = 0.9) to convert all the hydrogen sulfite present to SO2. Then the sample stream is conducted to the heat bath at 90 °C to cause the decomposition of adducts between sulfur dioxide and carbonyl compounds. When the sample stream arrives at the gas diffusion cell, the SO2 diffuses through the hydrophobic membrane of Teflon and is collected by the acceptor solution (sulfuric acid). Finally, the stream arrives at the reaction unit containing formaldehyde and p-rosaniline at pH 0.9 promoting the reaction with SO2 forming a p-rosaniline-sulfite complex whose optical density is measured.
Results and discussion
Validation of the method
The procedure establishes that turbid samples must be filtered or centrifuged; since all samples appeared clear and without turbidity, all samples were analysed without any previous treatment. To check the performance of the measurement procedure, a control solution, containing stabilized sulfite at a concentration of 80 mg L−1, was measured every day by triplicate.
For the validation of the system, linearity, limits of detection and quantification, precision and accuracy were evaluated according to the “Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics” [25].
Firstly, the linearity of the calibration curve was calculated using nine concentration levels (0.5, 1.0, 5.0, 10, 20, 50, 100, 150 and 200 mg L−1), prepared by appropriate dilution of the 1000 mg L−1 of the stock standard solution in water, by triplicate analysis. An excellent linearity was obtained (R2 = 1.000).
The limit of detection (LOD) and quantitation (LOQ), calculated as 3 and 10 times Sa/b respectively (“Sa” being the standard deviation of the intercept and “b” the slope of the calibration curve) were 0.60 and 2.00 mg L−1, respectively. These values demonstrated that enough sensitivity was achieved since the LOQ was five times lower than the limit established by regulations to label bottles as “contain sulfur dioxides”. Furthermore, these values are lower than those obtained with the official method recommended by the AOAC and the OIV [26,27,28].
The precision of the method was evaluated employing four wine reference materials: two white wines, one red wine and one sweet wine, with concentrations ranging between 50 and 190 mg L−1. The precision was calculated by measuring ten replicates of each reference wine on the same day (intra-day precision) and on different days during a week (inter-day precision). As it can be seen in Table 1, the results obtained were satisfactory with the relative standard deviation (RSD %) below 1.3% and 3.8% for intra-day and inter-day precision respectively. It is worth noting that the obtained relative standard deviations are lower than the precision values reported by other authors when using the official methods [26,27,28]. Furthermore, the Horwitz equation was also employed as an additional criterion for checking the precision of the method. The equation is RSD (%) = 21–0.5logC, being C the mass fraction expressed as a power of 10. For intra-day precision, the obtained values for RSD (%) would be lower than 1/2 (21–0.5logC), whereas in the case of inter-day precision, the values for RSD (%) would be lower than 2/3 (21–0.5logC). As observed, the values of RSDs obtained with the proposed methodology were highly satisfactory because, in all reference materials, the results achieved of RSD (%) for intra-day and inter-day precision did not exceed the limits calculated by the Horwitz equation.
Accuracy was checked employing nine wine reference materials: three white wines, three red wines, one rosé wine, one sweet white, and one aromatized wine. The range of concentrations of sulfur dioxide in the reference materials ranged from 42 to 190 mg L−1. Table 2 shows the mean and the standard deviation values obtained for the nine reference material with the proposed method (n = 10) and the assigned value, estimated through proficiency testing programs, together with the corresponding tolerance values. As can be seen, in all cases mean values measured in the automatic analyser are in good agreement with the values of the reference materials. After statistical evaluation by applying a paired t-test at 95% confidence, the calculated t was 0.73, which is lower that the t critical value (2.36). Furthermore, from the comparison of the values obtained by the automatic analyser and those of the reference materials, a lineal relationship was established, with R2 higher than 0.99 (Fig. 2). The 95% confidence interval calculated for the slope (0.96–1.15) and intercept (−11.9–5.8) did not differ significantly from the values of 1 and 0 respectively. These results demonstrated that the automatic sequential analyser provided excellent results, in agreement with the declared values of the reference materials.
Analysis of samples
The content of total sulfur dioxide was determined in 37 samples: 34 white wines and three red wines. All samples presented sulfur dioxide concentration below the values permitted by the regulation (150 mg L−1 for red wines and 200 mg L−1 for white wines). In all cases, the total sulfur dioxide concentration was higher than 10 mg L−1, which implies that all the bottles must be labelled “contain sulfur dioxides” according to legislation.
The concentration of sulfur dioxide ranged from 30 to 186 mg L−1, with a mean content of 95 mg L−1. The majority of the analysed samples presented sulfur dioxide concentration below 150 mg L−1, except for one white wine (186 mg L−1). For red wines, this content ranged from 52 to 98 mg L−1, whereas this content was higher in white wines, ranging from 30 to 186 mg L−1. Most of the analysed white wines (88%) had concentrations ranging between 69 and 120 mg L−1, while 9% had concentration above this value.
The residual levels found are all in the same range reported in Spain and other European countries. The obtained values in this study were similar to those reported in Spanish wines (33–128 mg L−1) [14] and in wines from five European countries (46–161 mg L−1) [17]. However, the levels of sulfur dioxide obtained in the analysed samples were lower than those reported for wines from Brazil (< 10.0–235 mg L−1) [27] and for wines from South Korea (< LOD-341 mg L−1) [28].
Additionally, to assess the quality of the obtained results, the content of SO2 was also analysed in the 37 commercial samples by p-rosaniline method employing a segmented flow analyser, which is an accredited method (ISO 17025) in the Laboratorio Agrario e Fitopatolóxico de Galicia for the determination of SO2 in wines. For quantification purposes, seven working solutions of concentrations 5.0, 10, 20, 50, 100, 150 and 200 mg L−1 were prepared by appropriate dilution of the 1000 mg L−1 of the stock standard solution. As can be seen in Fig. 3, a good correlation was achieved between the results obtained by both methodologies with a high regression coefficient (R2 = 0.97). The 95% confidence interval calculated for the slope (0.95–1.07) and intercept (−5.0–6.5) included the values of 1 and 0, respectively. The results obtained with both methods were also compared by a Bland Altman plot (Fig. 4) showing methods did not differ significantly from 0 (95% confidence interval from −7.3–10.1), being the differences only due to random errors. Therefore, the results provided for both methods were statistically comparable.
Conclusions
In this work, a sequential automatic analyser was validated to determine directly the content of sulfur dioxide in wines based on the reaction with 5,5′-dithiobis (2-nitrobenzoic acid) followed by photometric determination of the chromogenic generated. The method was validated in terms of linearity, limits of detection and quantitation, precision and accuracy, achieving satisfactory results.
As an application, 37 samples of wines from Galicia (NW Spain) were analysed, showing that the concentration of sulfur dioxide in all samples, was below the maximum permitted level set by European Legislation. The proposed method provided results statistically comparable to those obtained by the widely used p-rosaniline method by using a segmented flow analyser.
In short, the proposed methodology can be used successfully for rapid and accurate determination of sulfur dioxide in wines permitting, as well as, an output rate of approximately 50 samples per hour. Therefore, it could be established as a suitable protocol for routine quality control to determine total sulfur dioxide towards compliance with EU directives. Furthermore, it can be considered a green analytical tool because in comparison with the reference methodologies, the proposed procedure decreases both the reagent consumption and waste generation.
Data availability
All data generated during this study are included in this published article.
References
OIV International Organization of Vine and Wine (2021) SO2 and wine. A review. https://www.oiv.int/sites/default/files/2022-09/oiv-collective-expertise-document-so2-and-wine-a-review_en.pdf. Accessed 25 October 2023
European food safety authority (2016) Scientific opinion on the re-evalution of sulfur dioxide (E 220), sodium sulfite (E 221), sodium bisulfite (E 222), sodium metabisulfite (E 223), potassium metabisulfite (E 224), calcium sulfite (E 226), calcium bisulfite (E 227) and potassium bisulfite (E 228) as food additives. EFSA J 14(4):4438. https://doi.org/10.2903/j.efsa.2016.4438
Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off J Eur Union L 354:16–33. http://data.europa.eu/eli/reg/2008/1333/oj
OIV International Organization of Vine and Wine (2020) Review of practices for the reduction of SO2 doses used in winemaking. Resolution OIV-OENO 631-2020. https://www.oiv.int/standards/review-of-practices-for-the-reduction-of-so2-doses-used-in-winemaking. Accessed 25 October 2023
Commission Delegated Regulation (EU) 2019/934 of 12 March 2019 supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council as regards wine-growing areas where the alcoholic strength may be increased, authorised oenological practices and restrictions applicable to the production and conservation of grapevine products, the minimum percentage of alcohol for by-products and their disposal, and publication of OIV files. Off J Eur Union L 49:1–50. https://data.europa.eu/eli/reg_del/2019/934/oj
OIV International Organization of Vine and Wine (2017) List of OIV admitted compounds and their status as additives and processing aids and the use levels or residual limits. Resolution OIV-OENO 567A-2016. https://www.oiv.int/public/medias/4954/oiv-oeno-567a-2016-en.pdf. Accessed 25 October 2023
Joint FAO/WHO Expert Committee on Food Additives (2000) Evaluation of certain food additives. World Health Organization, Series No 891, Geneva. https://iris.who.int/bitstream/handle/10665/42245/WHO_TRS_891.pdf?sequence=1. Accessed 25 October 2023
Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Off J Eur Union L 304:18–63. http://data.europa.eu/eli/reg/2011/1169/oj
U.S. Food and Drug Administration. Code of Federal Regulations Title 21. Part 101 Food labeling. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=101&showFR=1&subpartNode=21:2.0.1.1.2.7. Accessed 25 October 2023
Commission Regulation (EC) No 607/2009 of 14 of July 2009 laying down certain detailed rules for the implementation of Council Regulation (EC) No 479/2008 as regards protected designations of origin and geographical indications, traditional terms, labelling and presentation of certain wine sector products. Off J Eur Union 193, 60–139. http://data.europa.eu/eli/reg/2009/607/oj
AOAC International. Association of Official Analytical Chemists (2023) Method 990.28 sulfites in foods. Optimized Monier–Williams method. Official methods of analysis, 22th edn. Arlington
OIV International Organization of Vine and Wine (2023) Total sulfur dioxide (titrimetry). Method OIV-MA-AS323-04A2. Compendium of international methods of wine and must analysis. https://www.oiv.int/standards/annex-a-methods-of-analysis-of-wines-and-musts/section-3-chemical-analysis/section-3-2-non-organic-compounds/section-3-2-3-other-non-organic-compounds/total-sulfur-dioxide-%28titrimetry%29-%28type-ii%29. Accessed 25 October 2023
OIV International Organization of Vine and Wine (2023) Sulfur dioxide (Iodometry). Method OIV-MA-AS323-04B. Compendium of international methods of wine and must analysis. https://www.oiv.int/standards/annex-a-methods-of-analysis-of-wines-and-musts/section-3-chemical-analysis/section-3-2-non-organic-compounds/section-3-2-3-other-non-organic-compounds/sulfur-dioxide-%28iodometry%29-%28type-iv%29. Accessed 25 October 2023
García-Guzmán JJ, Hernández-Artiga MP, Palacios-Ponce de León L, Bellido-Milla D (2015) Selective methods for polyphenols and sulphur dioxide determination in wines. Food Chem 182:47–54. https://doi.org/10.1016/j.foodchem.2015.02.101
Oliveira SM, Lopes TMIS, Tóth IV, Rangel AOSS (2009) Development of a gas diffusion multicommuted flow injection system for the determination of sulfur dioxide in wines, comparing malachite green and pararosaniline chemistries. J Agric Food Chem 57:3415–3422. https://doi.org/10.1021/jf803639n
Gómez-Otero E, Costas M, Lavilla I, Bendicho C (2014) Ultrasensitive, simple and solvent-free micro-assay for determining sulphur dioxide preservatives (E220–228) in foods by HS-SDME and UV–vis micro-spectrophotometry. Anal Bioanal Chem 406:2133–2140. https://doi.org/10.1016/j.foodchem.2017.02.026
Aberl A, Coelhan M (2013) Determination of sulfur dioxide in wine using headspace gas chromatography and electron capture detection. Food Addit Contam A 30(2):226–233. https://doi.org/10.1080/19440049.2012.743191
Koch M, Köppen R, Siegel D, Witt A, Nehls I (2010) Determination of total sulfite in wine by ion chromatography after in sample-oxidation. J Agric Food Chem 58:9463–9467. https://doi.org/10.1021/jf102086x
Molinero-Abad B, Alonso-Lomillo MA, Domínguez-Renedo O, Arcos-Martínez MJ (2014) Sulfite oxidase biosensors based on tetrathiafulvalene modified screen-printed carbon electrodes for sulfite determination in wine. Anal Chim Acta. https://doi.org/10.1016/j.aca.2013.12.041
Schneider M, Türke A, Fischer WJ, Kilmartin PA (2014) Determination of the wine preservative sulphur dioxide with cyclic voltammetry using inkjet printed electrodes. Food Chem 159:428–432. https://doi.org/10.1016/j.foodchem.2014.03.049
Giménez-Gómez P, Gutiérrez-Capitán M, Puig-Pujol A, Capdevila F, Muñoz S, Tobeña A, Miró A, Jiménez-Jorquera C (2017) Analysis of free and total sulfur dioxide in wine by using a gas-diffusion analytical system with pH detection. Food Chem 228:518–525. https://doi.org/10.1016/j.foodchem.2017.02.026
Moreira Goncalves L, Grosso Pacheco J, Magalhaes PJ, Rodrigues JA, Araújo Barros A (2010) Determination of free and total sulfites in wine using an automatic flow injection analysis system with voltammetric detection. Food Addit Contam A 27(2):175–180. https://doi.org/10.1080/19440040903261547
OIV International Organization of Vine and Wine (2018) Summary of resolutions adopted in 2018 by the 16th general assembly of the OIV. Resolution OIV-OENO 600-2018. https://www.oiv.int/summary-of-resolutions-adopted-in-2018-by-the-16th-general-assembly-of-the-oiv. Accessed 25 October 2023
EBC Analytica. European Reference Analytical Methods for Breweries. Method 9.25.3-total sulfur dioxide in beer: p-Rosaniline method (IM) 2005, 23 October 2018. https://brewup.eu/ebc-analytica/beer/total-sulphur-dioxide-in-beer-p-rosaniline-method-im/9.25.3. Accessed 25 October 2023
Magnusson B, Örnemark U (eds) (2014) Eurachem guide: the fitness for purpose of analytical methods—a laboratory guide to method validation and related topics, 2nd edn. Available from www.eurachem.org
Cressey P, Jones S (2009) Levels of preservatives (sulfite, sorbate and benzoate) in New Zealand foods and estimated dietary exposure. Food Addit Contam A 26(5):604–613. https://doi.org/10.1080/02652030802669188
Donato Machado RM, Figueiredo Toledo MC, Vicente E (2009) Sulfite content in some Brazilian wines: analytical determination and estimate of dietary exposure. Eur Food Res Technol 229:383–389. https://doi.org/10.1007/s00217-009-1071-7
Cho YS, Kim JJ, Jeon G, Chung MS, Joo Y, Lee KW (2021) Total SO2 levels and risk assessment of wine and fruit wine consumed in South Korea Yong. Food Control 127:108124. https://doi.org/10.1016/j.foodcont.2021.108124
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
J.F. Campo-Martínez: Formal analysis, investigation, validation, visualization. M.L. Enseñat-Berea: Supervision, validation. J. Fernández-Paz: Conceptualization, review and editing, project administration. M.J. González-Castro: Supervision, validation, writing original draft, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to be declared.
Ethics requirements
This article does not contain any studies using humans or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Campo-Martínez, JF., Enseñat-Berea, ML., Fernández-Paz, J. et al. Validation of a fast automated photometric method for the analysis of sulfur dioxide in wines. Eur Food Res Technol (2024). https://doi.org/10.1007/s00217-024-04496-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00217-024-04496-x