Abstract
Carotenoids have a wide range of applications in the food, pharmaceutical, and cosmetic industries as natural coloring agents and antioxidants. Consequently, industries are more concerned about extracting them from natural resources and by-products. The present research aimed to evaluate the extraction efficiency of carotenoids from orange peels using hydrophobic deep eutectic solvents (HDESs) as alternatives for organic solvents. The antioxidant capacity and color stability of HDESs extracts were monitored for 20 days and to intensify the extraction process, ultrasound-assisted extraction (UAE) was optimized using a response surface methodology (RSM). Menthol:Eucalyptol (Me:Eu) extract showed the highest carotenoid extraction yield [359.3 ± 3.5 mg/100 g of fresh weight (fw)], and also presented high stability during the storage period. HDESs extracts showed higher antioxidant capacity compared to hexane extracts, while Me:Eu extracts showed the lowest color variation (5.9 ± 0.2). Optimal parameters using Me:Eu were extraction time of 20 min, ultrasonic power of 120 W (60%), and solid–liquid ratio of 1:20 (g/mL) reaching a carotenoid content of 573.4 mg/100 gfw. While, C12:C8 optimal parameters were 10 min, 80 W (40%), and a solid–liquid ratio of 1:10 (g/mL), providing a carotenoid content of 183.7 mg/100 gfw. To establish if the solvents used are greener alternatives, the EcoScale was used and showed that UAE is a sustainable method to recover carotenoids using HDESs. Overall, the results showed that HDESs can improve carotenoid stability, and when combined with the intrinsic safety and edibility of their components, it makes these extracts appealing for food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids are a class of natural pigment formed from eight isoprene molecules and is one of the most important antioxidant compounds [1]. These molecules can be classified in carotenes as lycopene and β-carotenes, and xanthophylls as β-cryptoxanthin and lutein. There has been reported that astaxanthin has 100 times more antioxidant activity than vitamin E [2]. In consequence, the extraction of carotenoids from natural resources has lately attracted interest for their high antioxidant power. Additionally, the revalorization of food by-products as new sources of bioactive compounds is a tendency and is in compliance with sustainable development trends [3]. Considering the high production of orange (Citrus sinensis) juice, peels are an abundant waste that can be useful to extract valuable compounds. Orange peels are composed of flavedo and albedo; flavedo presents the majority of carotenoids [4].
Nowadays, the chemical industry is more focused on the development of environmentally friendly methods for separating and extracting natural ingredients from raw materials [5]. In this way, the most crucial step in every extraction is to use several solvents and experimental settings to maximize the extraction efficiency. Most traditional extraction techniques for carotenoids use organic solvents as acetone, hexane, chloroform, acetonitrile, methanol, etc., which can contribute to pollution and can be harmful. Also, the stability of carotenoids in organic solvents during extraction or storage has been proved to be poor [1]. The new challenge for the industry is finding novel extraction methods and solvents that are extremely efficient, cost-effective, and environmentally friendly.
Deep eutectic solvents (DESs), including hydrophilic and hydrophobic DESs, have recently undergone improvements in their use in the extraction of various natural actives [6,7,8,9,10]. The majority of synthesized DESs are miscible with aqueous media and hydrophilic organic solvents, which allows the extraction of more polar compounds [11,12,13,14]. In contrast, hydrophobic deep eutectic solvents (HDESs) are directed to extract lipophilic compounds to broaden this scope of uses. To create the HDESs, long-chain quaternary ammonium salts were initially melted with weakly water-soluble carboxylic acids for the first time in 2015 [15]. Due to their lower vapor pressure and improved efficiency, HDESs have gained more attention as a potential replacement for volatile organic solvents in the extraction of hydrophobic molecules [9, 10, 16,17,18]. The selection of the appropriate extraction method, after choosing the suitable solvent, is also a key step for optimal extraction. One of the most effective, least expensive, and straightforward extraction methods currently in use is ultrasound-assisted extraction (UAE), and it is frequently used in conjunction with DESs as solvent [9, 19]. In this regard, a combination of environmentally friendly, biodegradable, and sustainable solvents, such as HDESs, with a highly effective extraction technology, such as UAE, is promising since it may boost solvent penetration after being subjected to ultrasonic cavitation.
Based on the information presented above, and considering the physicochemical properties of carotenoid molecules, specifically, their non-polar and fat-soluble nature, HDESs were selected for its extraction from orange peels. Therefore, the aims of this study were: (i) to evaluate the HDESs efficiency to extract carotenoids from orange peels, (ii) to evaluate the antioxidant and color stability of HDESs extracts during storage, (iii) to optimize the recovery of carotenoids from orange by-product using HDESs associated with UAE method using response surface methodology (RSM), (iv) to investigate this approach as a green extraction alternative for the recovery of valuable bioactive compounds using green metrics. The results in this study can provide new green alternative solvents for carotenoid extraction from plant materials.
Materials and methods
Materials
The orange peels were obtained from orange fruits (Citrus sinensis, Navel cultivar) donated by a local agricultural cooperative (Carlet, Spain). Oranges were washed and stored in plastic bags at – 20 °C, until further use. Menthol (purity > 99%), lauric acid (purity > 98), octanoic acid (purity > 99%), and eucalyptol (purity > 99%) were purchased from Sigma (St. Louis, MO, USA). Red Nile, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl (DDPH) were purchased from Sigma-Aldrich (Missouri, USA). Hexane, methanol, ethanol, and acetonitrile were purchased from J.T Baker Chemical Co. (Deventer, Netherlands).
HDESs preparation and characterization
HDESs was prepared as described by Dai et al. [20]. The components were placed in a bottle without the addition of water. Subsequently, they were stirred and heated at 50 °C for 1 h in a Shaker-Incubator ES-20/60 (Biosan, Riga, Latvia) until a transparent liquid was obtained. The mixtures used were menthol:eucalyptol (Me:Eu) and lauric acid:octanoic acid (C12:C8). For HDESs characterization, the polarity was measured and compared with other organic solvents. Nile red probe was used following the method of Jeong et al. [21]. For all solutions, a specific λmax was obtained from the corresponding spectrum. The molar transition energy (ENR) was calculated using the following Eq. 1. Solvents with higher polarity have higher shifts λmax, which means lower ENR values.
Extraction process
Briefly, 1 g of milled peels was added to 10 mL of HDESs or with organic solvents (hexane, methanol, ethanol, and acetonitrile). The traditional extraction was done by magnetic stirring and heating at 45 °C for 30 min. At the same time, UAE was performed by an ultrasonic processor Q500 (Qsonica, USA) with a working frequency fixed at 20 kHz and 200 W at 45 °C for 30 min. For the temperature control, the extraction was performed in ice and the temperature was controlled using a thermometer. Extractions were performed in three replicates and were filtered and stored in amber vials at 4 °C until analysis.
Determination of total carotenoid
For the determination of total carotenoid (TC) content of the extracts, the method of Ordoñez-Santos et al. [22] was followed using a GENESYS 10S UV–Vis spectrophotometer (Thermo Fisher Scientific, USA) using the respective solvent used for extraction as blank for each extract. The total carotenoid content of the extracts was expressed as mg β-carotene/100 g of fresh weight and was calculated using the following Eq. 2:
where A is the absorbance at 450 nm, V is the total volume, ɛ is the extinction coefficient (2560), and P is the weight (g) of sample.
Total antioxidant capacity (DPPH method) and extracts stability
The assay was performed following the method described by Brand-Williams et al. [23]. The reaction was performed by adding 50 μL of extracts or solvent to 1.45 mL of DPPH-colored radical (0.06 mM). Then the sample was incubated for 30 min at room temperature. The absorbance was measured at the wavelength of 515 nm and the percentage of inhibition (% I) was calculated using Eq. 3. The Trolox standard was prepared over the range of 0–0.5 mM for the calibration curve and the results were expressed as micromoles of Trolox equivalents (μM TE/mL) of HDESs. For monitoring the antioxidant stability of the extracts and solvents, samples were exposed to ambient light for 20 days at room temperature. Then the antioxidant activity was determined every 5 days as described above.
Color determination and stability
The color measurements were analyzed by considering the CIELAB color system (L*, a*, b*) using a Hunter Labscan II spectrophotometric colorimeter (Hunter Associates Laboratory Inc., Reston, VA., U.S.A) and following the recommendations of the Commission Internationale de d’Eclairage (CIE 2004). The CIELAB color space is represented by three scalar parameters or Cartesian coordinates: L*, lightness, which varies from 0 (absolute black) to 100 (absolute white); a*, associated with changes in redness, and b*, associated with changes in yellowness–blueness. Color was measured every 5 days during 20 days in presence of light and the difference in color (ΔE) was calculated following Eq. 4:
Optimization process
The optimization parameters for total carotenoids from orange peels using HDESs and UAE were performed by an RSM. A three-level and three-factor, Box–Behnken design consisting of fifteen experimental runs was employed including four replicates at the center point. The extraction variables were the ultrasonic intensity (X1, 20–60%), extraction time (X2, 10–30 min), and solvent-to-solid ratio (X3, 10–20 mL/g). The actual and coded levels of the independent variables are given in Table 1. The Design-Expert program (11.0 version) was used to analyze the data, the F test was used to interpret the coefficients and a quadratic model was used to build response surfaces. By analyzing the lack of fit, coefficient of determination (R2), and Fisher test value (F value) obtained from the analysis of variance, the suitability of the model was assessed (ANOVA). By maintaining one response variable at its optimal level and comparing it against two factors, three-dimensional response surface plots were created (independent variables) and just the ones with significant correlation (p < 0.05) are presented. After obtaining the mathematical models, the optimization of all responses was performed to maximize the total carotenoid content. The experimental sequence was randomized to minimize the effects of external factors. A quadratic polynomial equation was used to develop an experimental model that correlates the responses to independent variables. A generalized second-order polynomial Eq. 5 was used:
where y is total carotenoids, β0, βi, βii, and βij are the regression coefficients for linear, quadratic, and interaction terms, respectively, and xi e xj are the independent variables.
Finally, the validation of the suggested optimal UAE conditions for each solvent was performed to verify the repeatability of models, comparing the predicted values with the experimental data using relative error (RE) in Eq. 6:
Green metric tool
To analyze the environmental effect,the EcoScale described by Van Aken et al. [24] was used. This method considers penalty points (PP) to reduce from a 100% environmentally safe process, according to various parameters, with tabulated PP values. The EcoScale combines parameters such as the use of hazardous solvents, energy consumption, yield, and economic aspects. For this analysis, the TE and UAE were compared using HDESs and hexane for carotenoid extraction. The results were ranked on a scale from 0 to 100 using the following scores: > 75, excellent; > 50, acceptable; and < 50, inadequate.
Statistical analysis
Three replicates were included, and the results were reported as mean ± standard deviation (SD). The ANOVA was performed to verify the significant differences in the parameters studied concerning the sample analyzed, and the factors included. Subsequently, a Tukey test (p < 0.05) was applied to identify differences at each level.
Results and discussion
HDESs characterization
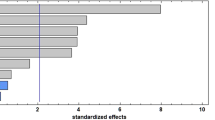
According to the ten-step method developed by Jin et al. [25] for screening new solvents, the first step is to identify the conventional solvent to be replaced and consequently select potential replacement candidates. The affinity of the solvents for the target compounds is usually related to physicochemical characteristics; for this reason, the polarity values using the Red Nile probe, expressed in ENR, were determined in HDESs and compared to organic solvents and the results are shown in Fig. 1. When the extraction is performed using traditional methods, the solvent should have a constant polarity and only the target compound that is dissolved can be extracted [5]. As can be observed, lower ENR values indicate higher polarity. HDESs are comparable to less polar solvents, such as acetonitrile or hexane. For this reason, it is expected that carotenoids present affinity with HDESs, and their extraction can be more effective. The ENR values of HDESs vary between 52.3 and 54.0 kcal/mol [26], and this is in good agreement with the results obtained for Me:Eu and C12:C8 in the present study. However, in the case of Me:Eu, it presented a lower polarity close to the value obtained for acetonitrile. This result is similar as that reported by Rebocho et al. [27], where a mixture made by menthol also showed lower polarity as acetonitrile and dichloromethane. In contrast, the mixture of C12:C8 showed an intermediate polarity that can be assessed for the saturated aliphatic chain of its constituent fatty acids. Viñas-Ospino et al. [19] also described that HDESs had similar polarities than hexane, accordingly it is expected that the further results using HDESs are efficient for carotenoid extractions. Lipid-soluble materials, such as carotenoids, are typically extracted using more volatile organic extractants including acetonitrile, ethanol, hexane, and methanol. However, because of their volatility and toxicity, the mentioned organic solvents are a risk to both, the environment and the workers. As a result, research into environmentally friendly extraction solvents is gaining popularity and HDESs have been referred to as good substitutes for organic solvents in recent years [28]. Also, they are considered very safe for human beings and eco-friendly because they are readily degradable in aquatic environments [29].
Total carotenoid content in traditional and UAE
The extraction of carotenoids was performed by traditional extraction (TE) and UAE to compare the efficiency of HDESs as solvent using these extraction methods. The results are presented in Fig. 2. There was significant difference (p < 0.05) between TE using HDESs and organic solvents, where Me:Eu and C12:C8 showed the higher extraction yields and methanol the lowest. However, applying UAE, the extract obtained with Me:Eu showed the highest carotenoid content (359.3 ± 3.5 mg/100 gfw (fresh weigh)) and from the solvents was the hexane extract (174.4 3 ± 3.8 mg/100 gfw). Other studies had reported similar results, where using ultrasounds, the efficiency is greatly improved, yielding higher values of carotenoids [3, 30]. Additionally, the hexane demonstrated similar polarity than HDESs and as was expected more efficient extraction of carotenoids. In contrast, ethanol and methanol showed higher polarities and the results demonstrated a lowest extraction yield. The benefits of using ultrasound for vegetal extraction include greater mass transfer, improved solvent penetration, lower extraction temperatures, quicker extraction rates, and higher product yields [3]. However, temperature is a parameter that should be taken into account due to the interaction and degradation of carotenoids during the extraction [9]. Another important point to consider is that UAE is a more environmentally friendly process than maceration. In this way, UAE can emit 200 g of CO2/100 g of extracted solid material into the atmosphere compared to 3600 g of CO2/100 g of extracted solid material emitted in maceration [31].
Me:Eu showed the highest extraction yield of carotenoids using UAE; for this reason, the capacity of reuse was assessed by the extraction efficiency (EE%) and results are shown in Fig. 3. In the first cycles, Me:Eu extracted 85% of carotenoids and about 33.5% in the last cycle. The loss of solvent per cycle was around 15% and the supernatant solvent was used for five consecutive cycles demonstrating the great potential of reusing HDESs. However, it can be observed that the extraction capability of Me:Eu decreased when the frequency of cycles increased, due to the reduced dissolution of carotenoids in HDESs, compared to fresh solvent [7].
Antioxidant activity and extracts stability
The bioactivity of carotenoids mainly depends on their solvent environment; thus, it is necessary to study the antioxidant activity in HDESs [1, 32]. For this reason, one of the objectives of this work was to evaluate the behavior of the carotenoids from orange peels in the HDESs extracts. For this purpose, the antioxidant activity of the extracts was evaluated by the DPPH radical scavenging method and their stability during ambient light exposure for 20 days. Since hexane showed better carotenoid extraction yields from the other organic solvents, it was chosen as control for the further antioxidant and stability tests. The results for the antioxidant capacity of HDESs mixtures, and HDESs extracts after storage are shown in Fig. 4. It can be observed that HDESs have the antioxidant capacity by themselves, as their components also have [33]. HDESs extracts showed higher antioxidant capacity when compared to hexane extracts, and this is in good agreement with the previous section, where HDESs extracts also showed high extraction of carotenoids. A satisfactory correlation was found to associate the carotenoid concentration with antioxidant activity (R2 = 0.97). This can be explained with the antioxidant properties of carotenoids that can provide a hydrogen atom to reduce the free radical DPPH to a stable form. It has been reported before that HDESs based on menthol were more efficient protecting carotenoids and their antioxidant capacity [1]. Based on the presented results, it can be indicated that the antioxidant activity of carotenoids is enhanced by HDESs compared to organic solvents. DES can improve not only the antioxidant activity, but also the biological effects of bioactive compound extracts [34]. Viñas-Ospino et al. [19] reported that an extract made by menthol:camphor and orange peels showed a low cell viability in tumor cells (26.7%). Additionally, the medical properties of terpenes have been demonstrated before, where they can decrease tumor size, inhibit the growth of cancerous cells, reduce cholesterol levels, and exhibit a biocidal effect on microorganisms in vitro [35].
Color stability
The degradation of carotenoids is the principal cause of the color variations in HDESs extracts. To further understand, the ΔE was monitored during ambient light exposure for 20 days in HDESs extracts and hexane extract as control. The visual appearance of the mixtures after storage is shown in Fig. 5. The investigated extracts showed no apparent signs of precipitation, layering, or phase separation over the course of storage, suggesting that the HDESs mix are generally stable. The initial colors of HDESs extracts containing carotenoids were light orange and luminous yellow. And it should be mentioned that the HDESs extracts were still clear and transparent after storage at 25 °C for 20 days, indicating the satisfactory stability of carotenoids in the HDESs [1].
The results for the color stability and ΔE are presented in Table 2, they show a clearly different color in C12:C8 extract and hexane extract after storage. An increase in L* and a significant decrease in a* and b* were observed when increasing storage time as reported by Li et al. [1] When ΔE is less than 3, it is called JND or “just notable difference” which can be translated as a barely perceptible difference [36]. As can be observed in both HDESs extracts and hexane ΔE was more than 3, however the extract with Me:Eu showed the lowest ΔE (5.9 ± 0.2). The difference in color changes might be explained by the decreasing contents of carotenoids in the HDESs extracts. The stability of carotenoids in HDESs has been reported before by Stupar et al. [9] and can be related to the hydrogen bonding interactions between solutes and solvent molecules.
Optimization process
The choice of extraction technique and its parameters has a crucial role in the successful recovery of bioactive compounds. For this reason, the optimization of the experimental parameters reflects on the possible implementation at the industrial level and influences the costs and the process efficiency. The previous results have demonstrated the efficiency of UAE and HDESs for carotenoid extraction from orange peels, and the subsequent paragraphs will explain the optimization process using Me:Eu and C12:C8 as extractant solvents. Using RSM-based optimization, it was possible to reduce the quantity of solvent and sample needed for trials, save time and effort, and determine the best conditions for maximizing carotenoid extraction. Different combinations of the three factors: extraction time (X1), ultrasound power (X2), and solid–liquid ratio (X3) were assessed. Table 3 displays the independent variables, where 15 experimental runs were performed randomly according to the UAE experimental conditions. The software used the results to determine the model fitness using ANOVA, and the F test and the results are presented in Table 4. The p values must be less than 0.05 to guarantee the proposed model fitness at the 95% confidence level. The created model has shown to be significant for carotenoid extraction based on the p values reported, using both solvents. The experimental values were within the confidence interval, and the standard error predicted was small, further supporting the model’s accuracy. The three independent variables, X1, X2, and X3, applied important effects on the total carotenoid. Nevertheless, X3 (solvent-to-solid ratio) does not have any influence on the extraction yield based on the model just using Me:Eu solvent. Experimental data by RSM models for each of the responses are expressed based on the below Eqs. 7 and 8:
Optimal parameters for maximization of total carotenoid extraction using Me:Eu were extraction time of 20 min, ultrasonic power of 60% (120 W), and solvent-to-solid ratio of 20 mL/g of orange peel reaching a carotenoid content of 573.4 mg/100 gfw. While extracting using C12:C8, the optimal conditions were extraction time of 10 min, ultrasonic power of 40% (80 W), and solvent-to-solid ratio of 10 mL/g of orange peel providing a carotenoid content of 183.7 mg/100 gfw. By choosing a short extraction time in both cases, the high levels of oxidative degradation and isomerization of carotenoids can be prevented, as well as the production of free radicals that can occur by cavitation and high temperatures [37].
The predicted experimental variables (total carotenoid content) matched well according to the low dispersion of points presented in Fig. 6. Then the optimization was validated by UAE at the optimized conditions where the TC was higher. The observed and predicted values, with relative error (RE), for each variable were:
-
(1)
TC mg/100 g (C12:C8) = 183.7 ± 1.2 (observed), 182.2 (predicted), RE (%) = 0.85
-
(2)
TC mg/100 g (Me:Eu) = 573.4 ± 3.5 (observed), 556.6 (predicted), RE (%) = 3.02
All measured values felt within the 95% confidence interval. As a result, the optimal conditions were confirmed, proving that the suggested models can accurately predict the total carotenoid contents using Me:Eu and C12:C8 as solvents.
Effect of extraction parameters on UAE
According to the previously presented results, Me:Eu and C12:C8 HDESs using UAE showed promising carotenoid extraction results. The extraction efficiency is strongly dependent on several experimental extraction parameters. Therefore, extraction time, ultrasound (US) power, and solvent-to-solid ratio were investigated. A total of 15 experimental points were investigated and listed in Table 3. Following the proposed models previously presented, three-dimensional (3D) response plots were displayed (Fig. 7) to indicate the interaction between the two variables and the response values.
First, Fig. 7-a1 presents the plots using Me:Eu as the solvent, presenting the response surface generated by the power and time on carotenoid extraction, where an increase in the US power and a decrease in the ratio can enhance the carotenoid extraction. This response may be explained by the physical cavitation processes created by the US, which alter the cell wall and make the pigment more soluble, enhancing the diffusion of carotenoids in the solvent [22]. However, it should be considered that UAE can increase the temperature and contribute to the degradation of carotenoids, and for this a short extraction time must be used [9, 38]. Figure 7-a2 shows the plot combining the US power and solvent-to-solid ratio, where an increase in the US power and a decrease in the ratio enhanced the extraction. One of the most important aspects to be considered for a green process is the minimization of solvent use and this agrees with our findings. Figure 7b shows the results using C12:C8 as solvent. Figure 7-b1 shows the interaction between the power and extraction time, where it followed the same tendency as using Me:Eu, while US power increases, higher yields of carotenoids can be obtained in a shorter extraction time. The quick extraction time needed to attain the best yield suggests that the target compound can easily be dissolved once the solvent has reached the plant matrix. Additionally, it is an interesting factor to consider for industrial reasons because it can lower the energy required for the extraction process, which would probably result in lower costs and greater process sustainability [18]. Finally, in Fig. 7-b2, the relation between power and the solvent-to-solid ratio is presented. It shows that using high US power and increasing the solvent-to-solid ratio, carotenoid extraction yield is improved.
The recovery of carotenoids from orange peels may vary depending on different factors such as cultivars and variety [39], but it can also change due to the extraction technique applied and the experimental conditions. It also was noticed during the experiment, where total carotenoid content varied between 574.4 and 65.6 mg/100 gfw extracted by Me:Eu, while using C12:C8 was between 183.7 and 84.0 mg/100 gfw depending on the different experimental extraction conditions. Even though the research about using HDESs to extract carotenoids from orange peels is still limited, the results obtained can be compared with other green solvents. For example, Murador et al. [4], used ionic liquids to extract carotenoids from orange peels, which is also an environmentally friendly solvent. They used [BMIM+][Cl−] to obtain a TC content of 32.0 ± 2.1 μg/g. The use of HDESs is a significant advancement in the context of environmentally friendly chemistry and developing more methods to get crucial value-added compounds like carotenoids is, in fact, one of challenges to increase the sustainability of the chemical industry [40].
Green method evaluation: EcoScale
According to Chemat et al. [41], the proposed extraction method can be considered “Green”, if it employs food by-products, uses UAE, and the involves application of green solvents that increases yield, cuts down on processing time, solvent consumption, and unit costs operations. It is also possible to use the EcoScale, a tool that allows benchmarking and rating the procedures, to assess how environmentally friendly is the improved procedure [24]. This tool indicates great reaction conditions (EcoScale > 75), acceptable conditions (EcoScale > 50), or inadequate conditions (50 ≥ EcoScale) within a value ranging from 0 to 100. The technical setup, temperature, time, workup, and purification of a chemical reaction are all considered by this tool, together with all information on the reagents utilized (quantity, price, and safety) [42]. The results obtained using the EcoScale are presented in Table 5. Traditional and UAE extraction using hexane showed EcoScale values of 49.4 and 48.0, respectively, which demonstrate inadequate conditions. Using HDESs, the results showed > 50 in the EcoScale, which reveals an acceptable rate.
Following the environmental assessment, it is safe to recommend using UAE to recover carotenoids using HDESs solutions. Additionally, the two mixtures of natural and edible components approved by the Flavor and Extract Manufacturers Association (FEMA) as generally recognized as safe (GRAS) flavor ingredients (Eucalyptol FEMA Nº 2465; DL- menthol: FEMA Nº 266; octanoic acid: FEMA N◦ 2799; lauric acid: FEMA Nº 2614) were prepared in the presented research to provide an improvement for the use of HDESs as extractant solvents. With these results and the EcoScale benchmarks, the resultant extract can be used as a bioactive natural colorant without the need for solvent removal. HDESs have been also tested as a delivery system for bioactive ingredients in pharmaceutical or nutraceutical product applications [43].
Conclusion
The present results provide valuable information for carotenoid extraction from orange peels and the possible utilization of carotenoid-rich HDESs extracts for formulation in functional food products. HDESs showed higher extraction yields compared to organic solvents and using UAE, the extraction was intensified. The extracts also demonstrated an improved antioxidant capacity and enhanced color stability during storage. Even though, carotenoids showed better stability in Me:Eu extract during storage, it is important to mention that menthol, due to its high volatility, is easier to be recycled than traditional DESs to achieve cleaner production and reuse. Additionally, the applied extraction process showed to be a promising environmentally friendly extraction method from the point of view of green chemistry. Due to the GRAS constituents of the mixtures, this study also sets off the possibility of using carotenoid extracts to improve antioxidant formulations that could be used for human consumption. Future research on the use of HDESs to extract bioactive compounds that are fat soluble is needed and should focus on their toxicological and biological effects. Also, the application of green extractions using HDES in industrial scale and solvent recyclability is still in early stages. The current work offers an alternative for a more environmentally friendly carotenoid extraction method from a by-product, as orange peel, and a green solvent, HDESs.
Data availability
Not applicable
Abbreviations
- ANOVA:
-
Analysis of variance
- C12:
-
Lauric acid
- C8:
-
Octanoic acid
- ΔE:
-
Difference in color
- DESs:
-
Deep eutectic solvents
- ENR :
-
Molar transition energy
- %EE:
-
Extraction efficiency
- FEMA:
-
Flavor and extract manufacturers association
- GRAS:
-
Generally recognized as safe
- HDESs:
-
Hydrophobic deep eutectic solvents
- JND:
-
Just notable difference
- Me:
-
Menthol
- Eu:
-
Eucalyptol
- PP:
-
Penalty points
- RE:
-
Relative error
- RSM:
-
Response surface methodology
- SD:
-
Standard deviation
- TC:
-
Total carotenoid
- TE:
-
Traditional extraction
- US:
-
Ultrasound
- UAE:
-
Ultrasound-assisted extraction
References
Li Y, Hu K, Huang C, Hu Y, Ji H, Liu S, Gao J (2022) Improvement of solubility, stability and antioxidant activity of carotenoids using deep eutectic solvent-based microemulsions. Colloids Surf B Biointerfaces 217:112591. https://doi.org/10.1016/J.COLSURFB.2022.112591
Nootem J, Chalorak P, Meemon K, Mingvanish W, Pratumyot K, Ruckthong L, Srisuwannaket C, Niamnont N (2018) Electrospun cellulose acetate doped with astaxanthin derivatives from Haematococcus pluvialis for in vivo anti-aging activity. RSC Adv 8(65):37151–37158. https://doi.org/10.1039/C8RA08156E
de Ferreira LMMC, Pereira RR, de Carvalho FB, Santos AS, Ribeiro-Costa RM, Carréra Silva Júnior JO (2021) Green extraction by ultrasound, microencapsulation by spray drying and antioxidant activity of the tucuma coproduct (Astrocaryum vulgare mart.) almonds. Biomolecules 12:24–26. https://doi.org/10.3390/biom11040545
Murador DC, Braga ARC, Martins PLG, Mercadante AZ, de Rosso VV (2019) Ionic liquid associated with ultrasonic-assisted extraction: a new approach to obtain carotenoids from orange peel. Food Res Int 126:108653. https://doi.org/10.1016/j.foodres.2019.108653
Cao J, Su E (2021) Hydrophobic deep eutectic solvents: the new generation of green solvents for diversified and colorful applications in green chemistry. J Clean Prod 314:127965. https://doi.org/10.1016/J.JCLEPRO.2021.127965
Sportiello L, Favati F, Condelli N, Di Cairano M, Caruso MC, Simonato B, Tolve R, Galgano F (2023) Hydrophobic deep eutectic solvents in the food sector: focus on their use for the extraction of bioactive compounds. Food Chem 405:134703. https://doi.org/10.1016/J.FOODCHEM.2022.134703
Khare L, Karve T, Jain R, Dandekar P (2021) Menthol based hydrophobic deep eutectic solvent for extraction and purification of ergosterol using response surface methodology. Food Chem 340:127979. https://doi.org/10.1016/J.FOODCHEM.2020.127979
Silva YPA, Ferreira TAPC, Celli GB, Brooks MS (2019) Optimization of lycopene extraction from tomato processing waste using an eco-friendly ethyl lactate-ethyl acetate solvent: a green valorization approach. Waste Biomass Valorization 10(10):2851–2861. https://doi.org/10.1007/s12649-018-0317-7
Stupar A, Šeregelj V, Ribeiro BD, Pezo L, Cvetanović A, Mišan A, Marrucho I (2021) Recovery of β-carotene from pumpkin using switchable natural deep eutectic solvents. Ultrason Sonochem 76:105638. https://doi.org/10.1016/J.ULTSONCH.2021.105638
Wang LT, Yang Q, Cui Q, Fan XH, Dong MZ, Gao MZ, Lv MJ, An JY, Meng D, Zhao XH, Fu YJ (2019) Recyclable menthol-based deep eutectic solvent micellar system for extracting phytochemicals from Ginkgo biloba leaves. J Clean Prod 244:118648. https://doi.org/10.1016/j.jclepro.2019.118648
Gómez-Urios C, Viñas-Ospino A, Puchades-Colera P, López-Malo D, Frígola A, Esteve MJ, Blesa J (2022) Sustainable development and storage stability of orange by-products extract using natural deep eutectic solvents. Foods 11(16):2457–2457. https://doi.org/10.3390/FOODS11162457
Gómez-Urios C, Viñas-Ospino A, Puchades-Colera P, Blesa J, López-Malo D, Frígola A, Esteve MJ (2023) Choline chloride-based natural deep eutectic solvents for the extraction and stability of phenolic compounds, ascorbic acid, and antioxidant capacity from Citrus sinensis peel. LWT Food Sci Technol. https://doi.org/10.1016/j.lwt.2023.114595
Panić M, Gunjević V, Cravotto G, Redovniković IR (2019) Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem 300:125185. https://doi.org/10.1016/j.foodchem.2019.125185
Wojeicchowski P, Ferreira AM, Abranches DO, Mafra MR, Coutinho AP (2020) Using COSMO-RS in the design of deep eutectic solvents for the extraction of antioxidants from rosemary. ACS Sustain Chem Eng 8:12132–12141. https://doi.org/10.1021/acssuschemeng.0c03553
Van Osch DJGP, Zubeir LF, Van Den Bruinhorst A, Rocha MAA, Kroon MC (2015) Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem 17:4518. https://doi.org/10.1039/c5gc01451d
Viñas-Ospino A, López-Malo D, Esteve MJ, Frígola A, Blesa J (2023) Green solvents: emerging alternatives for carotenoid extraction from fruit and vegetable by-products. Foods 12:863. https://doi.org/10.3390/foods12040863
Pitacco W, Samorì C, Pezzolesi L, Gori V, Grillo A, Tiecco M, Vagnoni M, Galletti P (2022) Extraction of astaxanthin from Haematococcus pluvialis with hydrophobic deep eutectic solvents based on oleic acid. Food Chem 379:132156. https://doi.org/10.1016/j.foodchem.2022.132156
Silva YPA, Ferreira TAPC, Jiao G, Brooks MS (2019) Sustainable approach for lycopene extraction from tomato processing by-product using hydrophobic eutectic solvents. J Food Sci Technol 56(3):1649–1654. https://doi.org/10.1007/s13197-019-03618-8
Viñas-Ospino A, Panić M, Bagović M, Radošević K, Esteve MJ, Radojčić Redovniković I (2023) Green approach to extract bioactive compounds from orange peel employing hydrophilic and hydrophobic deep eutectic solvents. Sustain Chem Pharm. 31:100942. https://doi.org/10.1016/j.scp.2022.100942
Dai Y, Witkamp GJ, Verpoorte R, Choi YH (2015) Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem 187:14–19. https://doi.org/10.1016/J.FOODCHEM.2015.03.123
Jeong KM, Ko J, Zhao J, Jin Y, Yoo DE, Han SY, Lee J (2017) Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J Clean Prod 151:87–95. https://doi.org/10.1016/J.JCLEPRO.2017.03.038
Ordóñez-Santos LE, Esparza-Estrada J, Vanegas-Mahecha P (2021) Ultrasound-assisted extraction of total carotenoids from mandarin epicarp and application as natural colorant in bakery products. LWT Food Sci Technol. https://doi.org/10.1016/j.lwt.2020.110598
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30
Van Aken K, Strekowski L, Patiny L, Strekowski L (2006) EcoScale, a semi-quantitative tool to select an organic preparation based on economical and ecological parameters. Beilstein J Org Chem. https://doi.org/10.1186/1860-5397-2-3
Jin S, Byrne F, McElroy CR, Sherwood J, Clark JH, Hunt AJ (2017) Challenges in the development of bio-based solvents: a case study on methyl (2,2-dimethyl-1,3-dioxolan-4-Yl) methyl carbonate as an alternative aprotic solvent. Faraday Discuss 202:157–173. https://doi.org/10.1039/c7fd00049a
Rodrigues LA, Pereira CV, Leonardo IC, Fernández N, Gaspar FB, Silva JM, Reis RL, Duarte ARC, Paiva A, Matias AA (2020) Terpene-based natural deep eutectic systems as efficient solvents to recover astaxanthin from brown crab shell residues. ACS Sustain Chem Eng 8(5):2246–2259. https://doi.org/10.1021/ACSSUSCHEMENG.9B06283/ASSET/IMAGES/LARGE/SC9B06283_0008.JPEG
Rebocho S, Mano F, Cassel E, Anacleto B, Bronze M, do R, Paiva A, Duarte ARC. (2022) Fractionated extraction of polyphenols from mate tea leaves using a combination of hydrophobic/hydrophilic NADES. Curr Res Food Sci 5:571–580. https://doi.org/10.1016/J.CRFS.2022.03.004
Van Osch DJGP, Dietz CHJT, Warrag SEE, Kroon MC (2020) The Curious case of hydrophobic deep eutectic solvents: a story on the discovery, design, and applications. ACS Sustain Chem Eng 8:10591–10612. https://doi.org/10.1021/acssuschemeng.0c00559
Dal Bosco C, Di Lisio V, D’Angelo P, Gentili A (2021) Hydrophobic eutectic solvent with antioxidant properties: application for the dispersive liquid-liquid microextraction of fat-soluble micronutrients from fruit juices. ACS Sustain Chem Eng 9(24):8170–8178. https://doi.org/10.1021/ACSSUSCHEMENG.1C01473
Benmeziane A, Boulekbache-Makhlouf L, Mapelli-Brahm P, Khaled Khodja N, Remini H, Madani K, Meléndez-Martínez AJ (2018) Extraction of carotenoids from cantaloupe waste and determination of its mineral composition. Food Res Int 111:391–398. https://doi.org/10.1016/j.foodres.2018.05.044
Saini A, Panesar PS, Bera MB (2021) Valuation of Citrus reticulata (kinnow) peel for the extraction of lutein using ultrasonication technique. Biomass Convers Biorefin 11(5):2157–2165. https://doi.org/10.1007/S13399-020-00605-4/FIGURES/7
Singh A, Ahmad S, Ahmad A (2015) Green extraction methods and environmental applications of carotenoids-a review. RSC Adv 5(77):62358–62393. https://doi.org/10.1039/c5ra10243j
Manurung R, Siregar AGA, Hasibuan R (2021) Menthol-carboxylic acid deep eutectic solvent for extraction of carotenoids from palm ester. Rasayan J Chem 14(3):1869–1874. https://doi.org/10.31788/RJC.2021.1436293
Yu J, Liu X, Zhang L, Shao P, Wu W, Chen Z, Li J, Renard CMGC (2022) An overview of carotenoid extractions using green solvents assisted by Z-isomerization. Trends Food Sci Technol 123:145–160. https://doi.org/10.1016/J.TIFS.2022.03.009
Martins MAR, Silva LP, Schaeffer N, Abranches DO, Maximo GJ, Pinho P, Coutinho AP (2019) Greener terpene−terpene eutectic mixtures as hydrophobic solvents. ACS Sustain Chem Eng 7:17414–17423. https://doi.org/10.1021/acssuschemeng.9b04614
Mezquita PC, Huerta BEB, Ramírez JCP, Hinojosa CPO (2015) Milks pigmentation with astaxanthin and determination of colour stability during short period cold storage. J Food Sci Technol 52(3):1634–1641. https://doi.org/10.1007/S13197-013-1179-4/FIGURES/3
Ricarte GN, Coelho MAZ, Marrucho IM, Ribeiro BD (2020) Enzyme-assisted extraction of carotenoids and phenolic compounds from sunflower wastes using green solvents. Biotech. https://doi.org/10.1007/s13205-020-02393-0
Viñas-Ospino A, Panić M, Radojčić-Redovniković I, Blesa J, Esteve MJ (2023) Using novel hydrophobic deep eutectic solvents to improve a sustainable carotenoid extraction from orange peels. Food Biosci 53:102570. https://doi.org/10.1016/j.fbio.2023.102570
Anticona M, Blesa J, Lopez-Malo D, Frigola A, Esteve MJ (2021) Effects of ultrasound-assisted extraction on physicochemical properties, bioactive compounds, and antioxidant capacity for the valorization of hybrid Mandarin peels. Food Biosci 42:101185. https://doi.org/10.1016/j.fbio.2021.101185
Chemat F, Vian MA, Cravotto G (2012) Green extraction of natural products: concept and principles. Int J Mol Sci 13(7):8615–8627. https://doi.org/10.3390/ijms13078615
Chemat F, Abert-Vian M, Fabiano-Tixier AS, Strube J, Uhlenbrock L, Gunjevic V, Cravotto G (2019) Green extraction of natural products. Origins, current status, and future challenges. Trends Analyt Chem 118:248–263. https://doi.org/10.1016/j.trac.2019.05.037
Benvenutti L, Zielinski AAF, Ferreira SRS (2022) Pressurized aqueous solutions of deep eutectic solvent (DES): a green emergent extraction of anthocyanins from a Brazilian berry processing by-product. Food Chem 13:100236. https://doi.org/10.1016/J.FOCHX.2022.100236
Silva E, Oliveira F, Silva JM, Reis RL, Duarte ARC (2021) Untangling the bioactive properties of therapeutic deep eutectic solvents based on natural terpenes. Curr Res Chem Biol. 1:100003. https://doi.org/10.1016/J.CRCHBI.2021.100003
Acknowledgements
This work was financially supported by the Ministry of Science and Innovation (Spain) -State Research Agency (PID-2019-111331RB-I00/AEI/10.13039/501100011033) and by “Generación Bicentenario” scholarship from the Ministry of Education of the Republic of Peru (PRONABEC). Agricultural Cooperative Sant Bernat from Carlet, Spain, donated the raw materials.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest to declare.
Compliance with Ethics
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Viñas-Ospino, A., López-Malo, D., Esteve, M.J. et al. Improving carotenoid extraction, stability, and antioxidant activity from Citrus sinensis peels using green solvents. Eur Food Res Technol 249, 2349–2361 (2023). https://doi.org/10.1007/s00217-023-04302-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04302-0