Abstract
The effects of hydrolysis by commercial food-grade proteases on the physicochemical and techno-functional properties of lentil protein concentrate were investigated. Lentil protein concentrate was hydrolysed with Alcalase, Novozym 11028 or Flavourzyme, and a control was prepared without enzyme addition under the same conditions. Differences in specificity between the three proteases were evident in the electrophoretic protein profile, reversed-phase HPLC peptide profile, and free amino acid composition. Alcalase and Novozym were capable of extensively degrading all the major protein fractions. Alcalase or Novozym treatment resulted in considerably higher solubility under acidic conditions compared to the control. Flavourzyme treatment resulted in moderately improved solubility in the acidic range, but slightly lower solubility at pH 7. Alcalase treatment resulted in slightly larger particle size and slightly higher viscosity. The foaming properties of the protein concentrate were not significantly affected by hydrolysis. Increased solubility in acidic conditions with hydrolysis could broaden the range of food and beverage applications for lentil protein concentrate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interest is currently growing in plant-based foods, especially alternatives to traditional animal-based products [1]. Animal-based foods contribute a large proportion of the protein intake for much of the world. However, their production typically results in greater environmental impacts compared to plant foods [2, 3]. Plant crops which have been primarily used for animal feeds are now receiving greater interest as protein sources for direct human consumption, potentially negating the relatively inefficient conversion of plant protein to animal protein [2, 4]. In addition to environmental concerns, consumers are also including more plant-based products in their diets for ethical, health and lifestyle-choice reasons [1]. However, many challenges remain, especially in formulating plant-based alternatives to traditional animal-based products. Potential challenges include poor nutritional, taste as well as techno-functional characteristics [1].
Pulse protein ingredients are receiving increased attention due to their useful techno-functional and nutritional properties, as potential alternatives to animal proteins and soy protein [5,6,7]. Pea protein isolates and concentrates are widely available commercially, and other pulse protein sources including lentil, faba bean and chickpea are also attracting increased interest. However, further development of processing is still necessary to optimise techno-functionality of such ingredients towards suitability for a range of products. Protein source, as well as processing, can have a major effect on ingredient techno-functionality [8, 9]. Even though pulse proteins generally provide useful techno-functionality compared to some other plant protein sources, there may still be a need for improvement in many cases, depending on the requirements [10, 11]. In particular, protein solubility tends to be poor in the mildly acidic range relevant for many food and beverage products, near the isoelectric point for most plant proteins [12, 13]. Enzymatic hydrolysis with proteases can be a very useful tool facilitating improved protein ingredients which meet the needs of specific applications [12, 14]. Changes in structural properties can provide improved techno-functionality such as solubility and interfacial properties [15]. Often the largest relative improvements are observed in mildly acidic conditions, around the isoelectric point, opening up new possible applications in this pH range [13, 15, 16]. Various studies have explored enzymatic hydrolysis as a tool to modify techno-functionality of different pulse proteins, including pea, chickpea and lupin proteins [17,18,19,20]. For pulse proteins generally, the impact of hydrolysis on techno-functional properties such as solubility, foaming and emulsification tends to vary considerably depending on factors such as protease, degree of hydrolysis and pH [17, 18]. It has been recognised for plant protein hydrolysate studies, in general, that information on surface properties, e.g. hydrophobicity and charge, is somewhat lacking and would be useful [13].
Lentil is now recognised as a source of protein with useful techno-functional as well as nutritional properties, with good potential for a number of food applications, including non-dairy high-protein beverages [21,22,23]. Some studies have explored the physicochemical and techno-functional properties of lentil protein hydrolysates [24,25,26], although less knowledge is available in this regard compared to more widely used proteins such as soy and pea protein. Regarding lentil protein techno-functionality, the effect of enzymatic hydrolysis on solubility has not been explored to a great extent, and the variety of proteases applied has been limited.
Alcalase and Flavourzyme have been commercially available for many years and have been widely studied with various substrates including pulse proteins [18, 19, 27]. On the other hand, information on Novozym 11028 is so far lacking in the scientific literature. Alcalase and Novozym are endopeptidases, acting on internal bonds of polypeptides. The main enzyme component of Alcalase, which is derived from a strain of Bacillus licheniformis, is subtilisin A, also referred to as Subtilisin Carlsberg [28, 29]. It is an alkaline serine protease, possessing a catalytic triad at the active site composed of serine, histidine and aspartic acid residues [29, 30]. Alcalase shows broad specificity; however, a preference is shown for sites with aromatic or hydrophobic residues [31, 32]. Two other endoproteases and one exopeptidase have been identified in Alcalase using mass spectrometry [33]. Flavourzyme, an enzyme mixture originating from Aspergillus oryzae, provides both exopeptidase and endopeptidase activity [34], although exopeptidase activity is often considered its primary function, potentially providing debittering or savoury flavour generation to hydrolysates. Various proteases have been identified in Flavourzyme, including aminopeptidases, carboxypeptidases, dipeptidyl peptidases and several endoproteases [33, 34]. Flavourzyme is often applied in conjunction with other proteases, e.g. Alcalase, as it can provide a debittering effect. In this study, the effects of hydrolysis using three commercial protease preparations, Alcalase 2.4 L FG, Flavourzyme 1000 L and Novozym 11028 on certain physicochemical and techno-functional characteristics of lentil protein concentrate are examined.
Materials and methods
Materials and chemicals
Alcalase® 2.4 L FG, Flavourzyme® 1000 L and Novozym® 11028 (referred to as Alcalase, Flavourzyme and Novozym) were kindly provided by Novozymes (Bagsværd, Denmark). All chemicals were purchased from Sigma-Aldrich (St Louis, Missouri, USA) unless otherwise stated.
Compositional analysis
Nitrogen content was analysed using the Kjeldahl method, and protein was calculated as N × 6.25. Fat content was analysed using the Soxhlet method, with petroleum ether as the extraction solvent. Ash content was analysed according to the AACC Method 08-01.01 [35]. Moisture content was analysed according to the AACC Method 44-17.01 [36]. Total starch was measured according to the AACC method 76-13.01 [37].
Production of lentil protein concentrate
Lentil protein concentrate (LPC) was prepared using red lentil flour as the input material, using the isoelectric precipitation method of Alonso-Miravalles et al. [38]; protein was extracted at pH 7.5, and insoluble fibre and starch were then separated by decanting. Lentil protein concentrate was recovered from the resulting protein extract by isoelectric precipitation. Subsequently, the precipitated proteins were separated in a disc separator and the sediment was neutralised with 3 M NaOH, pasteurised and spray dried to obtain the protein concentrate powder. All experiments were carried out using the same batch of protein concentrate produced at pilot scale.
Sample preparation and enzymatic hydrolysis
LPC powder was dispersed in distilled water to 5% (w/v) protein, to a total volume of 200 mL, using magnetic stirrer for 30 min at ~ 20 °C. This was followed by 15 min of mixing with an Ultra-Turrax T-10 equipped with an S10N-10G dispersing element (Ika-Werke GmbH, Staufen, Germany), while simultaneously mixing with the magnetic stirrer. Following this, samples were pre-heated in a water bath to 50 °C and pH was adjusted to and maintained at 7 with 0.5 M NaOH, using a Metrohm 907 Titrando auto-titrator and Tiamo® Titration Software (Metrohm AG, Herisau, Switzerland). Hydrolysis was started by adding 10 μL/g protein of the selected enzyme to the LPC dispersions. For 1 h, the samples were continuously stirred at 50 °C and the pH was maintained at 7. At the end of the enzymatic treatment, samples were heat treated at 85 °C for 15 min to ensure enzyme deactivation, followed by cooling to 20 °C, using an LP Electronic Mashing Device stirring water bath (Lochner Labor + Technik GmbH, Berching, Germany). The control sample was prepared using the same procedure but without enzyme addition. Samples were then stored overnight at 4 °C. Following equilibration to 20 °C, the pH was then checked and re-adjusted to 7 as necessary, and samples were analysed without further treatment unless otherwise stated.
Degree of hydrolysis
The degree of hydrolysis (DH) was determined by the o-phthalaldehyde (OPA) method described by Nielsen et al. [39]; the value htot, defined as the number of peptide bonds in the protein substrate (meq/g protein), was calculated as 7.31 from the amino acid composition of LPC. The DH result for the control sample was set to zero.
Electrophoretic protein profile
An Agilent Bioanalyzer 2100 Lab-on-a-Chip capillary electrophoresis system was used to analyse the protein profile and estimate molecular weights. Samples were prepared according to Amagliani et al. [40] with slight modifications: samples were mixed with SDS, thiourea and urea solution to give a concentration of 0.5% protein, 2% SDS, 2 M thiourea and 6 M urea, followed by shaking for 30 min at 20 °C. The samples were then centrifuged for 30 min at 3000 × g, 20 °C to remove insoluble material, and analysed using an Agilent Protein 80 kit according to the supplier instructions within the range 5–80 kDa. Molecular weight standards (ladder) were run simultaneously with samples. The sample buffer provided included upper and lower molecular weight markers. For reducing conditions, 1 M dithiothreitol (DTT) was added.
Reversed-phase high-performance liquid chromatography
Samples were mixed with trifluoroacetic acid (TFA) solution to give a final concentration of 4.5% protein and 0.1% TFA, vortexed, and centrifuged at 15,000 × g for 15 min at 20 °C, and filtered through 0.25 μm syringe filters. Samples were analysed by reversed-phase high-performance liquid chromatography (RP-HPLC) (Varian Pro Star instrument, model 230, Varian Associates Ltd., Walnut Creek, CA, USA) equipped with a Varian Pro Star UV detector (model 330, Varian Associates Ltd., Walnut Creek, CA, USA) with a C18 column (3.6 μm × 250 mm × 4.6 mm, Aeris Widepore, Phenomenex, UK). Gradient elution was carried out with a mixture of solvents containing 0.1% TFA in ultrapure water (solvent A) and 0.1% TFA in acetonitrile (ACN) (solvent B). Separations were performed using the following programme: isocratic elution of 5% B for 20 min, linear gradient from 5 to 50% B in 39 min, followed by an increment of B from 50 to 95% B in 5 min and a decrement of B from 95 to 5% in 30 min. Before injecting samples, the column was pre-equilibrated under the starting conditions for 10 min at a flow rate of 0.75 mL/min, column temperature 45 °C and detection at 214 nm. The sample injection volume was 40 μL.
Amino acid composition
Total amino acid analysis was carried out on LPC using ion chromatography with post-column ninhydrin derivatisation (fluorescence detection; UV detection for tryptophan) after adequate extraction and protein hydrolysis (separate hydrolysis procedures for the determination of tryptophan, sulphur-containing amino acids and remaining amino acids). Free amino acid analysis (without hydrolysis) was carried out on freeze-dried enzyme-treated samples as well as the control. This analysis was performed by Chelab S.r.l. (Resana, Italy), an accredited external analytical laboratory, and results are presented with uncertainty values.
Surface hydrophobicity
Surface hydrophobicity (S0) was measured based on the method of Hayakawa and Nakai [41] using 1-anilino-8-naphthalenesulphonate (ANS), with slight modifications as described by Karaca et al. [42]. Protein dispersions were serially diluted with 10 mM phosphate buffer (pH 7) in the range 0.0006–0.015% (w/v). ANS (20 μL; 8.0 mM in 0.1 M phosphate buffer, pH 7) was mixed with a 4 mL diluted sample and left in darkness for 15 min. Fluorescence was measured (λexcitation 390 nm, λemission 470 nm) and corrected by a blank measured without ANS. The results are presented as the slopes (r2 > 0.98) of the absorbance versus protein concentration.
Protein solubility
Samples were adjusted to pH 4, 5, 6 and 7 using HCl, then centrifuged at 4893 × g for 30 min at 20 °C. The protein contents (N × 6.25) of the original uncentrifuged samples and supernatants were measured using the Kjeldahl method. Protein solubility was expressed as follows:
Zeta potential
Zeta potential was determined using a Zetasizer nano-Z (Malvern Panalytical Ltd, Malvern, UK). Samples were diluted to 0.1% protein w/v in ultrapure water and pH was adjusted using HCl to 7, 6, 5 and 4, and centrifuged at 2000 × g for 10 min to remove any insoluble material. The zeta (ζ)-potential was measured at 20 °C for 120 s using an automatic voltage selection and zeta potential was calculated using the Smoluchowski model.
Particle size
Particle size distribution (PSD) was measured using a static laser light diffraction unit (Mastersizer 3000, Malvern Panalytical Ltd, Malvern, UK), covering a size range of 0.01–3000 µm, using a particle refractive index of 1.45, absorption of 0.001 and dispersant refractive index of 1.33. Samples were diluted 1:10 with ultrapure water before analysis, then introduced dropwise into the dispersing unit using ultrapure water as dispersant until a laser obscuration of ~ 12% was achieved. Data were presented in a volume-based PSD.
Rheological properties
The rheological behaviour was characterised using a controlled stress rheometer (MCR301, Anton Paar GmbH, Austria) equipped with a concentric cylinder measuring system (C-CC27-T200/SS, Anton Paar GmbH, Austria). The shear stress was measured as a function of shear rate ranging from 0.5 to 100 s−1. Apparent viscosity at 100 s−1 is referred to as viscosity. The measurements were carried out at 20 °C and the power law model was fitted from 13.6 to 100 s−1 to determine the flow behaviour index (n).
Foaming properties
Samples were frothed in 50 mL centrifuge tubes using an Ultra-Turrax T10 equipped with an S10N-10G dispersing element (Ika-Werke GmbH, Staufen, Germany) at speed 6 for 30 s. The foam height was measured after 3 min (as a clear interface was visible) and after 60 min. Foaming capacity and foam stability were calculated as follows:
Statistical data analysis
Compositional analyses were performed in triplicate unless otherwise stated, with data presented as mean ± standard deviation. Hydrolysates and control samples were produced in triplicate, and results are presented as the mean ± standard deviation of three batches. One-way analysis of variance (ANOVA) followed by Tukey's post hoc test (p < 0.05) was used to show significant differences, using IBM SPSS version 26 (Armonk, NY, USA).
Results and discussion
Composition of lentil protein concentrate
The nutritional composition of lentil protein concentrate is shown in Table 1. Overall, the composition was broadly similar to that previously observed for a lentil protein isolate produced using the same method, albeit with slight differences including lower protein content and higher ash content [38]. The amino acid profile for lentil protein concentrate is also shown in Table 1. This profile is broadly similar to those previously reported for lentil proteins, as well as legume seed proteins generally [22, 43, 44]. High contents of glutamic acid/glutamine, aspartic acid/asparagine, arginine and leucine were observed, while the concentrate was found to be relatively low in some indispensable amino acids, including sulphur containing amino acids and tryptophan.
Degree of hydrolysis and electrophoretic protein profile
The degree of hydrolysis (DH) for hydrolysed samples is shown in Table 2. Alcalase resulted in the highest DH, followed by Flavourzyme and Novozym, although the differences were relatively small.
There has been limited study of the structure and chemistry of lentil proteins specifically, while proteins of other seed legumes such as pea have been studied more extensively [45, 46]. At the same time, the storage globulins of legume seeds are broadly similar in structure [45]; therefore, a general structure is often referred to when describing the proteins of various pulses.
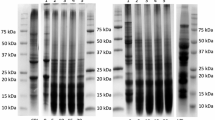
The protein profile (approximate molecular weight distribution) for lentil protein concentrate and hydrolysed samples is shown in Fig. 1. The protein profile is shown in gel format in Fig. 1a under reducing and non-reducing conditions for comparison. The molecular weight distribution of the control appears very similar to that of lentil protein isolates from a previous study [47]. For the control, the bands around 40 and 20 kDa appear slightly more intense under reducing compared to non-reducing conditions, suggesting dissociation of legumin into acidic and basic chains under reducing conditions. However, overall, the differences between reducing and non-reducing conditions appear minor. On the other hand, clear differences are apparent between the control and the different hydrolysed samples. Figure 1b shows the protein profile (under reducing conditions) in the electropherogram format, which allows a more detailed comparison. For the control, several major peaks can be observed. The peaks at 47.9, 52.7 and 56.8 kDa may correspond to vicilin subunits, which typically have a molecular weight of ~ 47–50 kDa, although considerable variability in size has been reported. The peaks at 22.3 and 39.3 kDa correspond to the typical molecular weights of the basic and acidic chains, respectively, of legumin [6, 28, 45]. At the same time, the exact molecular weights of the fractions may vary between different pulses. Jarpa-Parra et al. [46] purified legumin-like protein from lentil protein concentrate using rate-zonal centrifugation, and SDS-PAGE analysis revealed fractions of approximately 18, 20, 32, 42 and 47 kDa. The minor peaks around 15 kDa likely indicate the presence of low levels of albumins or γ-vicilin [28].
Representative electrophoretic protein profile of enzyme-treated samples and control. The size range is 5–80 kDa (represented by dashed lines). a Protein profile displayed in gel-type format under non-reducing and reducing conditions; C = control; A = Alcalase; N = Novozyme; F = Flavourzyme. b Protein profile displayed as electropherogram under reducing conditions
In general, significant degradation of proteins is evident for the enzymatically hydrolysed samples. More extensive degradation of the original protein peaks is apparent for Alcalase and Novozym compared to Flavourzyme. This was expected, as Alcalase and Novozym are endoprotease preparations, while Flavourzyme exhibits both exo- and endoprotease activity. It is likely that the exoprotease activity of Flavourzyme did not have a major impact on molecular weight. The different patterns of degradation observed in the protein profile indicate some differences in specificity between the 3 proteases. Flavourzyme treatment caused a reduction in height of the peaks in the range 35–60 kDa; however, no difference from the control was apparent for the 22.3 kDa peak. The inability to degrade the basic legumin subunit suggests narrower specificity for the endpoprotease components of Flavourzyme overall, or resistance of this subunit to proteolysis. Alcalase appeared capable of degrading all the protein fractions; only a small peak at 37 kDa remained of the proteins between 35 and 60 kDa, and only a small proportion of the peak at 22.3 kDa was still remaining. The most extensive degradation was observed with Novozym, even though the DH was slightly lower compared to Alcalase. A very small proportion of the 22.3 kDa peak was remaining, while above this molecular weight no peaks were visible, indicating that effectively all the proteins in this range were degraded to smaller peptides. For all three enzymes, some new peaks which were absent in the control, are visible in the 5–15 kDa range, most prominently for Novozym, followed by Alcalase and Flavourzyme. The results here are generally in agreement with previous studies regarding Alcalase and Flavourzyme. Alcalase, mainly the subtilisin A component, is known to exhibit broad specificity, being capable of hydrolysing a wide range of peptide bonds, but with a preference for those with adjacent hydrophobic residues [32]. Garcia-Mora et al. [28] found, using SDS-PAGE that Alcalase was capable of hydrolysing the major protein fractions in lentil protein concentrate to smaller peptides within 1 h at pH 8 and 40 °C. Rezvankhah et al. [44] found more extensive degradation of green lentil protein fractions with Alcalase compared to Flavourzyme. Similarly, for other substrates, such as lupin and pea protein, Alcalase has been shown to degrade a more extensive range of protein fractions compared to Flavourzyme [18, 19].
Peptide profile by reversed-phase high-performance liquid chromatography
Reversed-phase high-performance liquid chromatography (RP-HPLC) chromatograms are shown in Fig. 2. Peptides are separated mainly based on their hydrophobicity as well as molecular mass. Hydrophilic peptides are eluted first, while hydrophobic peptides are eluted later. Some differences can be seen in the peptide profiles between the samples, which indicates different specificities between the proteases. In the control, as well as hydrolysed samples, two major peaks are prominent, one in the hydrophilic region around 4 min and one in the hydrophobic region around 66 min. Besides these two major peaks, there are a limited number of small peaks visible. The hydrolysed samples share a somewhat similar profile, with many small peaks, predominantly in the hydrophobic region in the second half of the run, with most peaks eluting after 30 min. Although the overall profiles are somewhat similar, the peaks appear highest for the Novozym-treated sample, followed by Alcalase, and lowest for Flavourzyme. Similarly, the relatively large peak at the end of the run present in all samples is most prominent for Novozym, followed by Alcalase and Flavourzyme. Peaks nearer the beginning of the run appear more prominent for Flavourzyme and may correspond to free amino acids. Overall, it appears that a wide range of different peptides were generated. This could potentially have implications for the bitterness of the hydrolysates, as previous work has shown that bitter peptides tend to show a high degree of hydrophobicity [48]. In addition, for pulse proteins such as pea and lupin, higher perceived bitterness has been found for Alcalase hydrolysates compared to those of other proteases [18, 19].
Surface hydrophobicity
Surface hydrophobicity of proteins is an important property due to its influence on techno-functionality. The balance of exposed hydrophobic and hydrophilic regions of the proteins influences protein–protein and protein–water interactions, and therefore solubility. In addition, hydrophobic patches are required for surface activity of proteins; therefore, surface hydrophobicity is of interest due to its influence on emulsifying and foaming properties [25, 42]. Surface hydrophobicity is shown for the control and hydrolysed samples in Table 2. Values for Alcalase and Novozym hydrolysates were found to be significantly lower than the control, whereas no significant difference was found between Flavourzyme and the control. It is evident from the electrophoretic protein profile that greater structural changes/degradation occurred in the Alcalase and Novozym samples compared to Flavourzyme, which likely resulted in the observed difference in surface hydrophobicity. Some studies have shown that enzymatic hydrolysis leads to lower surface hydrophobicity compared to the intact protein substrate. Lentil protein hydrolysates produced with trypsin were shown to have lower surface hydrophobicity compared to the control sample [25]. Similarly, in the study of Xu et al. [26], hydrolysates of lentil, chickpea and pigeon pea protein produced with either Alcalase or bromelain, all showed lower surface hydrophobicity compared to the untreated samples. However, other studies have shown the opposite effect. Pea protein-enriched flour hydrolysed with either trypsin, Savinase, pepsin or papain had higher surface hydrophobicity compared to the untreated flour [49]. During hydrolysis and degradation of proteins into smaller peptides, previously buried hydrophobic regions can be exposed [13], potentially leading to increased surface hydrophobicity values. Where decreased surface hydrophobicity is observed following hydrolysis, it has been proposed that newly exposed hydrophobic regions may aggregate via hydrophobic interactions, and become re-buried within new structures [25, 50, 51].
Free amino acid profile
Free amino acid composition for hydrolysates and control is shown in Fig. 3. As expected, due to its exopeptidase activity, Flavourzyme treatment resulted in higher values for most amino acids compared to the control as well as the Alcalase and Novozym treated samples. For most amino acids, the Alcalase and Novozym hydrolysed samples did not differ majorly from the control. However, relatively high levels of cysteine were found for the Alcalase and Novozym samples, while it was not present in the control. Alcalase and Novozym treated samples also showed slightly higher values compared to the control for several amino acids, including tyrosine and phenylalanine. In addition, free glutamine was found for the Flavourzyme sample, but not for the control, Alcalase or Novozym samples. It appears that Alcalase and Novozym exhibited some exopeptidase activity, albeit relatively minor. Alcalase is produced from an extract from bacterial fermentation, and thus contains minor components other than Subtilisin A. In addition to Subtilisin, Merz et al. [33] identified 1 aminopeptidase and 2 endoproteases in Alcalase. Großmann et al. [52] found that hydrolysis of lupin protein isolate with Flavourzyme resulted in the generation of various free amino acids related to its exopeptidase activity, whereas hydrolysis with Alcalase contributed to negligible amounts of free amino acids in comparison. Großmann et al. [53] hydrolysed pea, soy and canola protein with six different proteases and found differences in bitterness and umami taste that could be related to the free amino acid profiles generated with each protease; in particular, umami taste was associated with higher levels of free glutamic acid and aspartic acid.
pH-dependent protein solubility and zeta potential
Protein solubility is a particularly important property for many food applications, as the contribution of a protein to various techno-functional properties (e.g. gelling, foaming, emulsifying) requires adequate solubility [13, 16, 54].
Protein solubility and zeta potential as a function of pH are shown in Fig. 4. The control follows the typical pattern for proteins of lentil or other pulses [8], with lowest solubility observed around pH 4–5 (near the isoelectric point), and increasing solubility moving towards neutral pH. This also relates to the zeta potential, where at pH 7 the highest value (28.6 mV) was observed, corresponding with the highest solubility (67.1%). The lowest zeta potential was observed at pH 5, corresponding to lowest solubility, although solubility at pH 4 was similarly low. This shows that surface charge is major determinant of solubility for the intact lentil protein, although other factors can also influence solubility including molecular size, charge distribution and hydrophobicity. Zeta potential for the Flavourzyme-treated sample followed the control quite closely, although there were some differences in solubility. Flavourzyme resulted in moderately higher solubility from pH 4–6, but slightly decreased solubility at pH 7. The solubility of both Alcalase- and Novozym-treated samples appeared to be much less influenced by pH. At neutral pH the Alcalase treated sample had similar solubility to the control, while Novozym resulted in a slight increase. For both, lowering pH towards 4 led to only a slight reduction in solubility, in contrast to the control. Zeta potential for both was similar to the control at pH 7 and 6 but deviated considerably approaching 4, with the isoelectric point shifting lower to pH ~ 4. Overall, the effect on pH-dependent solubility here may be expected, as typically, the largest relative improvements following enzymatic hydrolysis of proteins are observed near the isoelectric point where the original untreated protein demonstrates poor solubility [13]. Interestingly, Xu et al. [26] showed an overall negative effect on solubility for lentil protein when hydrolysed with Alcalase or bromelain, and also similar results for chickpea as well as pigeon pea protein. They found that for lentil protein, Alcalase increased solubility slightly at pH 4, but it was similar or lower across the rest of the pH range tested, while bromelain reduced the lentil protein solubility across the pH range. However, it was speculated that the assay used may not have been able to detect smaller peptides/amino acids. While the literature is somewhat lacking on solubility of lentil protein hydrolysates, it may be useful to compare with other pulse protein sources. García Arteaga et al. [18] examined the effects of hydrolysis with various enzymes on solubility of pea protein at pH 7 and 4.5. At neutral pH, solubility was either increased or decreased, depending on the enzyme used, along with minor differences due to hydrolysis duration. At pH 4.5, the untreated concentrate was very poorly soluble (2%) and every enzymatic treatment increased solubility. For Alcalase, solubility increased moderately at pH 7, but increased considerably at pH 4. For Flavourzyme, solubility was reduced at pH 7 but increased moderately at pH 4.5. This is broadly similar to the results observed in this study for Alcalase and Flavourzyme. For chickpea protein, Mokni Ghribi et al. [15] found that Alcalase improved solubility over a wide pH range (2–12), with the largest relative increase around pH 4, while solubility generally increased with higher DH. While in the current study Alcalase was found to provide a greater increase in solubility compared to Flavourzyme, Segura-Campos et al. [27] found the opposite effect for cowpea protein, where Flavourzyme increased solubility in the pH range of 2–10, while Alcalase only led to an increase at pH 4–6, and decreased solubility outside this range. Generally, the variation in effects due to different enzymes can be explained in part by differences in specificity, which leads to peptides with varying size and structural characteristics. In addition, different fractions of the protein substrate with varying solubility may be targeted, depending on the protease [17]. Furthermore, the enzyme deactivation step should not be overlooked as differences in heat treatment conditions may influence solubility. The increased solubility often observed after enzymatic hydrolysis has been attributed to decreased molecular weight, along with liberation of ionisable groups [13, 16, 55]. On the other hand, decreased solubility may also be observed, which may be attributable to newly exposed hydrophobic groups leading to aggregation and subsequent sedimentation [13]. The notable effect of hydrolysis here on solubility, especially with Alcalase and Novozym, was reduced sensitivity to changes in pH; even at minimum zeta potential only a slight reduction in solubility was observed. The relatively high solubility of the Alcalase and Novozym hydrolysates in this pH region is likely attributable to smaller average peptide size [12], while the Flavourzyme treatment yielded less extensive degradation of the main protein fractions. The shift to a lower isoelectric point for hydrolysates evident from the zeta potential, in this case for Alcalase and Novozym, has also been observed previously [15, 55]. This is suggestive of differences in the overall balance of exposed ionisable groups, corresponding to fewer exposed positively charged groups for Alcalase and Novozym hydrolysates at low pH.
Particle size distribution
Particle size of protein ingredients in dispersion is of interest, as it relates to solubility and stability. The presence of large particles tends to result in sedimentation. The particle size distribution and parameters are shown in Fig. 5 and Table 3, respectively. The control sample had a volume-weighted mean particle diameter (D[4,3]) of 12.31 µm. Slight differences from the control were apparent for the Novozym- and Flavourzyme-treated samples, with the Novozym showing a slightly narrower size distribution. Alcalase, on the other hand, resulted in a major increase in particle size, with the D[4,3] more than twice that of the other samples at 28.8 µm, while the upper range of the distribution extended to ~ 100 µm. Enzymatic hydrolysis can cause increased exposure of previously buried hydrophobic groups, leading to aggregation [55, 56]. It is possible that this occurred to a greater extent with Alcalase. The Alcalase hydrolysate may have been more susceptible to heat-induced aggregation during the protease deactivation step, compared to the other samples. However, the presence of considerably larger particles after Alcalase treatment did not result in reduced solubility compared to the control at neutral pH. It may be that the large particles do not represent a large proportion of the total protein, much of which may be present below the size range of the Mastersizer.
Rheological properties
The rheological behaviour of proteins in dispersion is important in, for example, high-protein beverages such as milk alternatives, where viscosity and flow behaviour influence mouthfeel [57]. Enzymatic hydrolysis may be useful for increasing solubility in these applications; however, changes in texture might occur which may or may not be desirable. The viscosity and flow behaviour index are shown in Table 4. Some slight differences were observed in the flow behaviour index, although the differences were relatively minor, and all samples could be considered to show approximately Newtonian behaviour. There were no significant differences in apparent viscosity between the Flavourzyme-treated sample and control, or between the Novozym treated sample and control, although the viscosity for Novozym was found to be significantly higher than that of Flavourzyme. The most notable difference was found for the Alcalase hydrolysate, which had significantly higher viscosity than the control. The higher viscosity for Alcalase might be due to the presence of larger particles. A higher effective particle volume fraction in the Alcalase treated sample might explain the higher viscosity [58]. Potentially, the size and morphology (e.g. less compact) of the particles may be conducive to increased interaction and therefore slightly higher viscosity. Hydrolysis with Alcalase has previously been shown to cause a reduction in viscosity for lentil protein isolate [24], as well as kidney bean protein isolate [59], although it should be noted that DH was considerably higher in those studies than the DH measured here. Other studies have used enzymatic hydrolysis to reduce viscosity of protein dispersions, which may be of particular interest where high-protein concentration is desirable, or where the protein ingredient generally gives highly viscous dispersions. Bajaj et al. [60] applied Alcalase, Flavourzyme or Neutrase, as well as some combinations thereof, to pea protein isolate in 15% dispersions. All the treatments reduced viscosity, to varying degrees. Lamsal et al. [61] found that hydrolysis of various soybean protein substrates with bromelain invariably led to major reductions in viscosity of 10% dispersions.
Foaming properties
Foaming capacity and foam stability are shown in Table 4. No significant differences were found between any of the samples for foaming capacity or foam stability, indicating that hydrolysis under these conditions had minimal effects on the foaming properties, regardless of the protease used, and the original foaming properties of the lentil protein were retained. Structural and surface properties of proteins, such as size, molecular flexibility and hydrophobicity influence their ability to form and stabilise foams [62]. Enzymatic hydrolysis can be used to modify these properties, thereby potentially improving foaming properties, and increased solubility may also correspond to improved foaming. At the same time, if the hydrolysis is too extensive, peptide length may be insufficient to form a strong interfacial layer and foam stability can be reduced [13]. Ahmed et al. [24] found that hydrolysis of lentil protein with Alcalase resulted in slightly increased foaming capacity, along with slightly reduced foam stability. Barac et al. [17] found that the foaming capacity and foam stability of pea protein either increased, decreased or remained unchanged depending on pea variety, protease, hydrolysis time and pH. In this study, it is apparent that hydrolysis did not impair or enhance foaming properties, despite major differences in the electrophoretic protein profile for hydrolysates compared to the control, in particular for Alcalase and Novozym.
Conclusions
Lentil protein concentrate was subjected to hydrolysis using either Alcalase, Novozym or Flavourzyme. Clear differences in specificity were found between the enzymes, particularly for Flavourzyme compared to Alcalase and Novozym, as evidenced by the electrophoretic protein profile, peptide profile and free amino acids released. Alcalase and Novozym both resulted in considerably increased solubility under acidic conditions, while Flavourzyme produced a more moderate improvement in the acidic range. The highest solubility was achieved using Novozym. Foaming properties were not significantly affected by hydrolysis. These results show that enzymatic hydrolysis can deliver significant improvement of solubility for lentil protein concentrate. This effect is most prominent in the acidic region where the untreated concentrate is poorly soluble. These enzymes could help bridge this gap in techno-functionality and open up new possibilities for lentil protein concentrates, for example application in mildly acidic products. The ability of the proteases, especially Alcalase and Novozym, to increase solubility could prove useful for the inclusion of lentil protein in high-protein beverages such as milk alternatives. These proteases allow retained solubility even at lower pH, which may be particularly relevant for acidic protein beverages e.g. drinkable-type yogurt products, or juices fortified with protein. Flavourzyme was somewhat less effective in increasing solubility; however, potential advantages regarding sensory properties may be important and could be explored in future studies. In addition, future work focusing on specific applications would be useful to further explore the potential of lentil protein hydrolysates. Novozym 11028 shows promise as a broad specificity endoprotease capable of increasing solubility and should be explored further.
References
McClements DJ, Grossmann L (2021) The science of plant-based foods: Constructing next-generation meat, fish, milk, and egg analogs. Compr Rev Food Sci Food Saf. https://doi.org/10.1111/1541-4337.12771
Poore J, Nemecek T (2018) Reducing food’s environmental impacts through producers and consumers. Science 360:987–992
Willett W et al (2019) Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 393:447–492
Day L (2013) Proteins from land plants—potential resources for human nutrition and food security. Trends Food Sci Technol 32:25–42
Fernando S (2021) Production of protein-rich pulse ingredients through dry fractionation: a review. LWT 141:110961
Singhal A, Karaca AC, Tyler R, Nickerson M (2016) Pulse proteins: from processing to structure-function relationships in Goyal A, ed. Grain Legumes. https://doi.org/10.5772/64020.London:IntechOpen
Sozer N, Holopainen-Mantila U, Poutanen K (2017) Traditional and new food uses of pulses. Cereal Chem J 94:66–73
Boye JI, Aksay S, Roufik S, Ribéreau S, Mondor M, Farnworth E, Rajamohamed SH (2010) Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res Int 43:537–546
Vogelsang-O’Dwyer M, Zannini E, Arendt EK (2021) Production of pulse protein ingredients and their application in plant-based milk alternatives. Trends Food Sci Technol 110:364–374
Can Karaca A (2021) Modification of legume proteins for improved functionality. IntechOpen, London. https://doi.org/10.5772/intechopen.96274
Fernando S (2021) Pulse protein ingredient modification. J Sci Food Agric. https://doi.org/10.1002/jsfa.11548
Panyam D, Kilara A (1996) Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci Technol 7:120–125
Wouters AGB, Rombouts I, Fierens E, Brijs K, Delcour JA (2016) Relevance of the functional properties of enzymatic plant protein hydrolysates in food systems. Compr Rev Food Sci Food Saf 15:786–800
Meinlschmidt P, Sussmann D, Schweiggert-Weisz U, Eisner P (2016) Enzymatic treatment of soy protein isolates: effects on the potential allergenicity, technofunctionality, and sensory properties. Food Sci Nutr 4:11–23
Mokni Ghribi A, Maklouf Gafsi I, Sila A, Blecker C, Danthine S, Attia H, Bougatef A, Besbes S (2015) Effects of enzymatic hydrolysis on conformational and functional properties of chickpea protein isolate. Food Chem 187:322–330
Eckert E, Han J, Swallow K, Tian Z, Jarpa-Parra M, Chen L (2019) Effects of enzymatic hydrolysis and ultrafiltration on physicochemical and functional properties of faba bean protein. Cereal Chem. https://doi.org/10.1002/cche.10169
Barac M, Cabrilo S, Stanojevic S, Pesic M, Pavlicevic M, Zlatkovic B, Jankovic M (2012) Functional properties of protein hydrolysates from pea (Pisum sativum, L) seeds. Int J Food Sci Technol 47:1457–1467
García Arteaga V, Apéstegui Guardia M, Muranyi I, Eisner P, Schweiggert-Weisz U (2020) Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov Food Sci Emerg Technol 65:102449
Schlegel K, Sontheimer K, Hickisch A, Wani AA, Eisner P, Schweiggert-Weisz U (2019) Enzymatic hydrolysis of lupin protein isolates—changes in the molecular weight distribution, technofunctional characteristics, and sensory attributes. Food Sci Nutr 7:2747–2759
Yust MDM, Pedroche J, Millán-Linares MDC, Alcaide-Hidalgo JM, Millán F (2010) Improvement of functional properties of chickpea proteins by hydrolysis with immobilised Alcalase. Food Chem 122:1212–1217
Jeske S, Bez J, Arendt EK, Zannini E (2019) Formation, stability, and sensory characteristics of a lentil-based milk substitute as affected by homogenisation and pasteurisation. Eur Food Res Technol 245:1519–1531
Khazaei H, Subedi M, Nickerson M, Martinez-Villaluenga C, Frias J, Vandenberg A (2019) Seed protein of lentils: current status, progress, and food applications. Foods 8:391
Verni M, Demarinis C, Rizzello CG, Baruzzi F (2020) Design and characterization of a novel fermented beverage from lentil grains. Foods 9:893
Ahmed J, Mulla M, Al-Ruwaih N, Arfat YA (2019) Effect of high-pressure treatment prior to enzymatic hydrolysis on rheological, thermal, and antioxidant properties of lentil protein isolate. Legume Sci 1:e10
Avramenko NA, Low NH, Nickerson MT (2013) The effects of limited enzymatic hydrolysis on the physicochemical and emulsifying properties of a lentil protein isolate. Food Res Int 51:162–169
Xu X, Qiao Y, Shi B, Dia VP (2021) Alcalase and bromelain hydrolysis affected physicochemical and functional properties and biological activities of legume proteins. Food Struct 27:100178
Segura-Campos MR, Espinosa-García L, Chel-Guerrero LA, Betancur-Ancona DA (2012) Effect of enzymatic hydrolysis on solubility, hydrophobicity, and in vivo digestibility in cowpea (Vigna unguiculata). Int J Food Prop 15:770–780
Garcia-Mora P, Peñas E, Frias J, Martínez-Villaluenga C (2014) Savinase, the most suitable enzyme for releasing peptides from lentil (Lens culinaris var. Castellana) protein concentrates with multifunctional properties. J Agric Food Chem 62:4166–4174
Tacias-Pascacio VG, Morellon-Sterling R, Siar E-H, Tavano O, Berenguer-Murcia Á, Fernandez-Lafuente R (2020) Use of Alcalase in the production of bioactive peptides: a review. Int J Biol Macromol 165:2143–2196
Polgár L (2005) The catalytic triad of serine peptidases. Cell Mol Life Sci 62:2161–2172
Adamson NJ, Reynolds EC (1996) Characterization of casein phosphopeptides prepared using alcalase: determination of enzyme specificity. Enzyme Microb Technol 19:202–207
Gupta R, Beg QK, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32
Merz M, Claaßen W, Appel D, Berends P, Rabe S, Blank I, Stressler T, Fischer L (2016) Characterization of commercially available peptidases in respect of the production of protein hydrolysates with defined compositions using a three-step methodology. J Mol Catal B Enzym 127:1–10
Merz M, Eisele T, Berends P, Appel D, Rabe S, Blank I, Stressler T, Fischer L (2015) Flavourzyme, an enzyme preparation with industrial relevance: automated nine-step purification and partial characterization of eight enzymes. J Agric Food Chem 63:5682–5693
AACC 08-01.01. Ash—Basic Method. AACC International Approved Methods of Analysis
AACC 44-17.01 Moisture—Air-Oven Method (Pulses). AACC Approved Methods of Analysis
AACC 76-13.01-Total starch assay procedure. AACC Approved Methods of Analysis
Alonso-Miravalles L, Jeske S, Bez J, Detzel A, Busch M, Krueger M, Wriessnegger CL, O’Mahony JA, Zannini E, Arendt EK (2019) Membrane filtration and isoelectric precipitation technological approaches for the preparation of novel, functional and sustainable protein isolate from lentils. Eur Food Res Technol. https://doi.org/10.1007/s00217-019-03296-y
Nielsen PM, Petersen D, Dambmann C (2001) Improved method for determining food protein degree of hydrolysis. J Food Sci 66:642–646
Amagliani L, O’Regan J, Kelly AL, O’Mahony JA (2017) Composition and protein profile analysis of rice protein ingredients. J Food Compos Anal 59:18–26
Hayakawa S, Nakai S (1985) Relationships of hydrophobicity and net charge to the solubility of milk and soy proteins. J Food Sci 50:486–491
Karaca AC, Low N, Nickerson M (2011) Emulsifying properties of chickpea, faba bean, lentil and pea proteins produced by isoelectric precipitation and salt extraction. Food Res Int 44:2742–2750
Gunes ZS, Can Karaca A (2021) Examining the amino acid composition, secondary structure, and physicochemical and functional properties of proteins isolated from local lentil landraces of Anatolia. Cereal Chem. https://doi.org/10.1002/cche.10446
Rezvankhah A, Yarmand MS, Ghanbarzadeh B, Mirzaee H (2021) Characterization of bioactive peptides produced from green lentil (Lens culinaris) seed protein concentrate using Alcalase and Flavourzyme in single and sequential hydrolysis. J Food Process Preserv 45:e15932
Boulter D, Croy RRD (1997) The structure and biosynthesis of legume seed storage proteins: a biological solution to the storage of nitrogen in seeds. In: Callow JA (ed) Advances in botanical research, vol 27. Academic Press, Cambridge, pp 1–92
Jarpa-Parra M, Bamdad F, Tian Z, Zeng H, Temelli F, Chen L (2015) Impact of pH on molecular structure and surface properties of lentil legumin-like protein and its application as foam stabilizer. Colloids Surf B: Biointerfaces 132:45–53
Joehnke MS, Jeske S, Ispiryan L, Zannini E, Arendt EK, Bez J, Sørensen JC, Petersen IL (2021) Nutritional and anti-nutritional properties of lentil (Lens culinaris) protein isolates prepared by pilot-scale processing. Food Chemistry: X 9:100112
Liu X, Jiang D, Peterson DG (2014) Identification of bitter peptides in whey protein hydrolysate. J Agric Food Chem 62:5719–5725
Konieczny D, Stone AK, Korber DR, Nickerson MT, Tanaka T (2020) Physicochemical properties of enzymatically modified pea protein-enriched flour treated by different enzymes to varying levels of hydrolysis. Cereal Chem 97:326–338
Jung S, Murphy PA, Johnson LA (2005) Physicochemical and functional properties of soy protein substrates modified by low levels of protease hydrolysis. J Food Sci 70:C180–C187
Paraman I, Hettiarachchy NS, Schaefer C, Beck MI (2007) Hydrophobicity, solubility, and emulsifying properties of enzyme-modified rice endosperm protein. Cereal Chem 84:343–349
Großmann KK, Merz M, Appel D, Fischer L (2019) A fast and novel approach to evaluate technical enzyme preparations for an efficient protein hydrolysis. Eur Food Res Technol 245:1695–1708
Großmann KK, Merz M, Appel D, Thaler T, Fischer L (2021) Impact of peptidase activities on plant protein hydrolysates regarding bitter and umami taste. J Agric Food Chem 69:368–376
Jiang Z-q, Pulkkinen M, Wang Y-j, Lampi A-M, Stoddard FL, Salovaara H, Piironen V, Sontag-Strohm T (2016) Faba bean flavour and technological property improvement by thermal pre-treatments. LWT Food Sci Technol 68:295–305
Klost M, Drusch S (2019) Functionalisation of pea protein by tryptic hydrolysis—characterisation of interfacial and functional properties. Food Hydrocolloids 86:134–140
Zhang Y, Zhou F, Zhao M, Ning Z, Sun-Waterhouse D, Sun B (2017) Soy peptide aggregates formed during hydrolysis reduced protein extraction without decreasing their nutritional value. Food Funct 8:4384–4395
McClements DJ, Newman E, McClements IF (2019) Plant-based milks: a review of the science underpinning their design, fabrication, and performance. Compr Rev Food Sci Food Saf 18:2047–2067
Berghout JAM, Boom RM, van der Goot AJ (2015) Understanding the differences in gelling properties between lupin protein isolate and soy protein isolate. Food Hydrocolloids 43:465–472
Al-Ruwaih N, Ahmed J, Mulla MF, Arfat YA (2019) High-pressure assisted enzymatic proteolysis of kidney beans protein isolates and characterization of hydrolysates by functional, structural, rheological and antioxidant properties. LWT 100:231–236
Bajaj PR, Bhunia K, Kleiner L, Joyner HS, Smith D, Ganjyal G, Sablani SS (2017) Improving functional properties of pea protein isolate for microencapsulation of flaxseed oil. J Microencapsul 34:218–230
Lamsal B, Jung S, Johnson L (2007) Rheological properties of soy protein hydrolysates obtained from limited enzymatic hydrolysis. LWT Food Sci Technol 40:1215–1223
Damodaran S (2005) Protein stabilization of emulsions and foams. J Food Sci 70:R54–R66
Acknowledgements
We would like to thank the following people for their invaluable expert advice, insight and technical assistance: Avril McCord, Andrea Hoehnel, Jairo Salas Garcia, Michael O’Grady and Dave Waldron. We would also like to thank the team at Novozymes for providing proteases as well as assistance, in particular Hans Peter Heldt-Hansen. In addition, we would like to thank Chelab S.r.l. for carrying out the amino acid analysis.
Funding
The work for this publication has been undertaken as part of the SMART PROTEIN project. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 862957.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This study does not involve research on human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vogelsang-O’Dwyer, M., Sahin, A.W., Bot, F. et al. Enzymatic hydrolysis of lentil protein concentrate for modification of physicochemical and techno-functional properties. Eur Food Res Technol 249, 573–586 (2023). https://doi.org/10.1007/s00217-022-04152-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04152-2