Abstract
In this study, the bioaccessible total phenolic compounds (TPC) and antioxidant capacity (AC) of commonly consumed bakery products enriched with raw (BH) or roasted (RBH) buckwheat hull were evaluated. The soluble and insoluble fractions obtained after in vitro enzymatic digestion of mixed rye/wheat bread with 4% of RBH (BRBH), wheat bread with 3% of BH (BBH), and their control counterparts were separated (C-BRBH and C-BBH, respectively). The addition of buckwheat hull, raw and roasted, significantly increased the values of analyzed parameters compared to control samples. Before the digestion, the highest values of TPC and AC were found for bread with 4% of RBH. After in vitro digestion of the bakery products, the content of TPC and AC in the soluble fraction was 75–90% higher compared to the values found in the undigested fraction. Generally, a decrease in the bioaccessibility index of enriched bakery products compared to control samples was observed. The obtained results indicate that buckwheat by-products may be used as a valuable ingredient for commonly used bakery products. Also, it was shown that the in vitro digestion model may be of relevance in assessing the bioaccessibility of bioactive compounds in commonly used bakery products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Buckwheat contains many nutrients and bioactive components that positively affect the human body as was presented by Kreft [1]. This pseudocereal is a good food source of nutritionally valuable components: protein with well-balanced amino acid composition, fiber, minerals, and in combination with other health-promoting components. Buckwheat is widely used in the world in various food products due to its proven hypotensive, hypocholesterolemic, hypoglycemic, neuroprotective, and antioxidant properties [2]. The main by-product during the obtaining of kasha or flour from buckwheat is the hull. From the literature data, it is known that buckwheat hull could be used as an additive in solid biofuel production [3, 4]. Also, there is some information on the application of these by-products to yogurt [5], noodles [6], or tea [7]. There are also studies on the use of an extract obtained from buckwheat hull in frozen-stored meat products [8] or mayonnaise [9], in both cases for the prolonged shelf life of products. As was indicated by Liu et al. [6], buckwheat hull has many already proven nutritional properties. It has more bioactive compounds than buckwheat flour, as presented by Zhang et al. [10]. Dziadek et al. [11] found in six selected cultivars and strains of common buckwheat (whole seeds, dehulled seeds, and hulls) the highest content of dietary fiber and total polyphenols as well as the highest antioxidant activity in the hulls compared to other examined samples. As presented in our previous investigations [12], commonly consumed bakery products can be enriched by the addition of buckwheat flour or hull and has a positive influence on the antioxidative potential as well as on the sensory and storage properties of the obtained products.
The antioxidant capacity of cereals or pseudocereals is difficult to measure because the compound responsible for this capacity is bound to the cell wall [13]. The main problem during the analysis of antioxidant capacity is encountered during the extraction of bioactive compounds from the food matrix, because their health benefits depend on bioaccessibility. Wojtunik-Kulesza et al. [14] in the review presented how the in vitro digestion conditions could influence the composition, bioaccessibility, and antioxidant activity of food polyphenols. The constituents which are released from the solid food matrix into a soluble phase may be expected to be more likely absorbable by the organism [15]. The bioavailability of bioactive compounds, which have high antioxidant activity, administered orally in pure form is much greater than the bioavailability of the same compounds from the food product matrix as presented by Dima et al. [16]. The method called QUENCHER, elaborated by Gökmen et al. [17], can be used to assess in vitro the total antioxidant capacity of solid foods or solid fractions after digestion without extraction. It enables evaluating the antioxidant activity in the residue portion obtained when food is exposed to in vitro intestinal digestion [18].
The review of Angelino et al. [19] showed potential strategies to improve phenolic bioaccessibility and bioavailability in bread. These strategies include the use of raw materials with high phenolic content or different processing technologies, such as fermentation or enzymatic treatment of ingredients. During in vitro hydrolysis of buckwheat products, Choi et al. [20] found that the baking process positively affects flavonoid digestibility and bioaccessibility. Whereas Świeca [21] showed a positive effect of elicitation, supported by the phenylpropanoids precursors, there was an increase in the phenolics content and antioxidant capacity of the potentially bioaccessible fraction of buckwheat sprouts.
This study aimed to investigate the effect of in vitro digestion of enriched commonly produced and consumed bakery products on the bioaccessible and non-bioaccessible total phenolic compounds (TPC) and antioxidant capacity (AC) content.
Materials and methods
Chemicals and reagents
Alpha-amylase, pepsin, pancreatin, bile salts, rutin, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), potassium persulfate, and 6-hydroxy- 2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Kits for assaying the antioxidant capacity of water-soluble substances (PCL ACW) were purchased from Analytik Jena (Leipzig, Germany). All other reagents were from Avantor Performance Materials Poland S.A. (Gliwice, Poland). Water was purified with a Mili-Q system (Millipore, Bedford, USA).
Buckwheat-enriched bakery product
All ingredients and procedures used for preparing bakery products were presented by Wronkowska et al. [12]. Briefly, commonly produced and consumed bakery products (in the northern part of Poland) were enriched with buckwheat hull: raw (BH) or roasted (RBH). In this study, wheat bread with 3% BH and rye/wheat bread with 4% RBH were used. The control samples were products made without the hull addition. Both types of buckwheat hulls were obtained from the common buckwheat (Fagopyrum esculentum Moench) during the dehulling process used in the preparation of unroasted and roasted buckwheat groats. Both hulls were milled into powder to particles < 400 μm before addition to bakery products (mill WZ-1, ZBPP, Bydgoszcz, Poland).
In vitro digestion of bakery products
The in vitro digestion was performed according to Delgado-Andrade et al. [22] using three steps to mimic digestion in the digestive tract: the mouth, stomach, and intestine. Fractions obtained after in vitro digestion, soluble and insoluble ones, were used for further analysis. The insoluble fraction was freeze-dried, and the soluble fraction was used in liquid form.
Determination of TPC and AC in bakery products before in vitro digestion
The extraction procedure and analysis of TPC content and AC (measured by ABTS) and photochemiluminescence (PCL) assay in bakery products before digestion were done according to Zieliński et al. [23].
Determination of TPC and AC in bakery products after in vitro digestion
The bioaccessible TPC and AC of the soluble fraction after digestion were evaluated according to Szawara-Nowak et al. [24]. The determination of non-bioaccessible TPC and non-bioaccessible AC of the insoluble fraction after sample digestion was conducted by the QUENCHER method as described by Szawara-Nowak et al. [22].
Statistical analysis
All types of analyzed samples were baked in two replications (3 loaves in each). Results are given as the means and the standard deviation of six independent measurements. To compare TPC and AC data obtained for buckwheat-enhanced bakery products and control samples, the analysis of variance (one-way ANOVA) was used. Also, the same analysis was done to compare data obtained before and after in vitro digestion of the examined bakery products. The Tukey test at a significance level of p < 0.05 was performed for post hoc comparison (Statistica 7.1, StatSoft, Kraków, Poland). Bioaccessible total phenolic compounds (TPC) and antioxidant capacity (AC) were assessed.
Results and discussion
In this study, commonly produced and consumed bakery products (wheat and mixed rye/wheat) before and after enrichment with buckwheat hull, raw (BH) or roasted (RBH) (by-products obtained during the buckwheat dehulling process), were analyzed for the content and bioaccessibility of total phenolic compounds and antioxidant capacity pre- and post-digestion in the in vitro model.
Total phenolic compound content before and after in vitro digestion
Before digestion, bakery products enriched by both used BH showed significantly higher TPC content compared to control samples (Table 1). The addition to the bread of roasted BH (BRBH) had a significantly greater influence on TPC content compared to the addition of raw BH (BBH). Li et al. [25] showed that extract from buckwheat hull exhibited higher TPC and AC compared to extract from buckwheat flour. Zhang et al. [10] found higher total phenolic content of buckwheat hull and bran compared to flour. Baking was reported to lead to a TPC decrease in wheat cookies enriched with buckwheat flour, but the TPC value of buckwheat cookies was higher compared to the wheat ones, as presented by Jan et al. [26].
In all the soluble fractions obtained after in vitro digestion, a significant increase of bioaccessible TPC compared to the samples before digestion was observed (Table 1). It was found that the insoluble solid fraction, resistant to enzymatic digestion, was still the source of non-bioaccessible TPC, which was still present in a sample but in a significantly lower amount compared to samples before digestion. Chandrasekara and Shahidi [27] found TPC increase after in vitro digestion of millet grains. The same observation was made by Gawlik-Dziki et al. [28] for the TPC of wheat bread under simulated digestion. Szawara-Nowak et al. [24] demonstrated that the TPC content was higher in the soluble fraction obtained after digestion of wheat bread enriched with buckwheat flour compared to the insoluble fraction. During the gastric phase of grain digestion, proteins are digested and some phenolic bound to them may be released as was shown by Chandrasekara and Shahidi [27]. But some parts of non-bioaccessible TPC may reach the colon unchanged, and in this part of the gastrointestinal tract may be transformed by the gut microbiota enzymes into a wide range of phenolic acids [29].
Antioxidant capacity before and after in vitro digestion
In our study, the AC of bakery products before and after in vitro digestion was analyzed using two assays: against ABTS+• radicals and against superoxide anion radicals (O2−•). Obtained data were shown in Table 1. Before digestion, the AC measured against ABTS+• radicals of the examined samples were as follows: C-RBH < RBH < C-BRBH < BRBH. Products BRBH and RBH showed significantly higher AC compared to control samples before digestion. After the in vitro digestion, the bioaccessible and non-bioaccessible AC significantly increased in all soluble and insoluble fractions obtained from the experimental bakery products. The ability of soluble fractions to scavenge ABTS+• radicals was more than 96% higher compared to samples before digestion. The highest value was found for the soluble fraction obtained from bread with 3% of raw BH (BBH) and its control (C-BBH), 66.81 and 67.28 μmol Trolox g−1 DM, respectively. After digestion, the insoluble fractions obtained from the buckwheat-enriched bakery products were still a source of compounds capable of scavenging ABTS+• radicals. In our study, the differences in the AC of the soluble and insoluble fraction were as large as those presented by Szawara-Nowak et al. [24] for wheat bread enriched with buckwheat flour. Baublis et al. [30] showed that simulated gastrointestinal pH treatments and enzymatic hydrolysis could increase the AC of wheat and wheat-based breakfast cereal extracts. Rufián-Henares and Delgado-Andrade [18] found that all the digestive processes occurring in the gastrointestinal tract could enhance the AC of grains, flour, or bread. As presented by Marques et al. [31], polyphenols may affect the activity of digestive enzymes through binding within the active site and changing their secondary structure. Phenolic compounds may also reduce the bile salts’ emulsification capacity.
The results presented in Table 1 showed that compared to the ABTS assay, lower values of the scavenging effect obtained by the PCL method were obtained. Before digestion, the highest AC measured by PCL ACW assays was observed in extracts from BRBH (1.23 μmol Trolox g−1 DM).
For all analyzed samples (control and buckwheat enriched) the increase of PCL ACW values in the soluble fraction, obtained after digestion, compared to samples before digestion was noticed. The ability to scavenge superoxide anion radicals was not determined in the insoluble fraction due to the limitations of the methodology. Chandrasekara and Shahidi [27] found that millet grain phenolics bound to the insoluble fiber could be released during in vitro gastrointestinal digestion. They also showed that different millet types could demonstrate different AC under the digestion conditions. The contents of polyphenols and thus the AC of extracts obtained during digestion of cereal samples were significantly higher compared to aqueous-organic extracts, as presented by Perez-Jimenez and Saura-Calixto [32]. This phenomenon suggested that the number of antioxidants released during digestion in the human intestines may be higher than could be expected from the data based on chemical extracts.
Potential bioaccessibility of TPC and AC of the examined bakery products
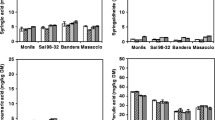
For better evaluation of the bioaccessibility of TPC and AC in vitro, the bioaccessibility index (BITPC, BIABTS, and BIPCL) was determined and defined as follows:
Similarly, the BI was analyzed for AC determined by ABTS and PCL ACW assays. BI value higher than 1 indicates high bioaccessibility, and lower than 1 indicates low bioaccessibility. Gawlik-Dziki et al. [33] introduced BI as a useful parameter to study the bioaccessibility of phenolics from coffee and coconut. As it was shown in Fig. 1, the values of BITPC, BIABTS, and BIPCL were higher than 1 for all bakery products studied, which is indicated by their high bioaccessibility. Generally, the BI values for all investigated enriched bakery products were lower compared to control samples. The only exception is the values obtained for the BITPC for bread BBH. For bread BRBH and its control, no statistically significant difference was found for the indicator BITPC and BIPCL. Hemery et al. [34] found that the amounts of bioaccessible phenolic acids were higher in whole-grain bread and bran-rich bread than in white bread, and the finer the bran particles in bran-rich bread, the more bioaccessible were the phenolic acids. However, Irakli et al. 35 showed that the amounts of bioaccessible phenolic acids released in vitro digestion of wheat bread with the addition of hop sourdough combined with rice bran were lower compared to the control sample.
The bioaccessibility index (BI) indicates the potential bioaccessibility of TPC and AC of the examined bakery products. BRBH bread with 4% of roasted buckwheat hull, C-BRBH control for BRBH, BBH bread with 3% of raw buckwheat hull, C-BBH control for BBH. Values with different letters (a,b) are significantly different; (p < 0.05), differences between the control and sample with buckwheat hull
Conclusions
Commonly consumed bakery products enriched with buckwheat by-products were subjected to in vitro digestion and the bioaccessible TPC and AC from soluble fraction obtained after digestion were determined, whereas the non-bioaccessible TPC and AC were investigated in the insoluble fraction. Also, for the examined products, the bioaccessibility index (BI) was calculated. Before digestion, the highest TPC among analyzed bakery products had the bread with 4% of roasted buckwheat hull (1.80 mg GAE g−1 DM). After in vitro digestion of the examined products, the content of TPC and AC in the soluble fraction was 75–90% higher as compared to the insoluble ones. The values of BI showed that enrichment with raw or roasted buckwheat hull (BH, BRH) does not affect the bioaccessibility of TPC and AC from bakery products. However, it was shown that the in vitro digestion model may be of relevance in assessing the bioaccessibility of bioactive compounds in bakery products.
References
Kreft M (2016) Buckwheat phenolic metabolites in health and disease. Nutr Res Rev 29:30–39
Yilmaz HӦ, Ayhan NY, Meriҫ ҪS (2020) Buckwheat: a useful food and its effects on human health. Curr Nutr Food Sci 16:29–34
Obidziński S, Piekut J, Dec D (2016) The influence of potato pulp content on the properties of pellets from buckwheat hulls. Renew Energy 87:289–297
Kulokas M, Praspaliauskas M, Pedišius N (2021) Investigation of buckwheat hulls as additives in the production of solid biomass fuel from straw. Energies 14:265
Znamirowska A, Sajnar K, Kowalczyk M, Kluz M, Buniowska M (2020) Effect of addition of spelt and buckwheat hull on selected properties of yoghurt. J Microbiol Biotechnol Food Sci 10(2):296–300
Liu D, Song S, Tao L, Yu L, Wang J (2022) Effects of common buckwheat bran on wheat dough properties and noodle quality compared with common buckwheat hull. LWT Food Sci Technol 155:112971
Zielińska D, Szawara-Nowak D, Zieliński H (2013) Antioxidative and anti-glycation activity of buckwheat hull tea infusion. Int J Food Prop 16:228–239
Hęś M, Szwengiel A, Dziedzic K, Le Thanh-Blicharz J, Kmiecik D, Górecka D (2017) The effect of buckwheat hull extract on lipid oxidation in frozen-stored meat products. J Food Sci 82:882–889
Park BI, Kim J, Lim T, Hwang KH (2019) Flavonoids in common and tartary buckwheat hull extracts and antioxidant activity of the extracts against lipids in mayonnaise. J Food Sci Technol 56(5):2712–2720
Zhang W, Zhu Y, Liu Q, Bao J, Liu Q (2017) Identification and quantification of polyphenols in hull, bran and endosperm of common buckwheat (Fagopyrum esculentum) seeds. J Funct Food 38:363–369
Dziadek K, Kopeć A, Pastucha E, Piątkowska E, Leszczyńska T, Pisulewska E, Witkowicz R, Francik R (2016) Basic chemical composition and bioactive compounds content in selected cultivars of buckwheat whole seeds, dehulled seeds and hulls. J Cereal Sci 69:1–8
Wronkowska M, Zieliński H, Szatowicz B, Ostaszyk A, Lamparski G, Majkowska A (2019) Effect of roasted buckwheat flour and hull enrichment on the sensory qualities, acceptance and safety of innovative mixed rye/wheat and wheat bakery products. J Food Process Preserv 43:e14025
Serpen A, Capuano E, Fogliano V, Gökmen V (2007) A new procedure to measure the antioxidant activity of insoluble food components. J Agric Food Chem 55:7676–7681
Wojtunik-Kulesza K, Oniszczuk A, Oniszczuk T, Combrzyński M, Nowakowska D, Matwijczuk A (2020) Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—a non-systematic review. Nutrients 12:1401. https://doi.org/10.3390/nu12051401
Šulniūtė V, Jaime I, Rovira J, Venskutonis PR (2016) Rye and wheat bran extracts isolated with pressurized solvents increase oxidative stability and antioxidant potential of beef meat hamburgers. J Food Sci 81:519–527
Dima C, Assadpour E, Dima S, Jafari SM (2020) Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr Rev Food Sci Food Saf. 19(6):2862–2884
Gökmen V, Serpen A, Fogliano V (2009) Direct measurement of the total antioxidant capacity of foods: the ‘QUENCHER’ approach. Trends Food Sci Technol 20:278–288
Rufián-Henares JA, Delgado-Andrade C (2009) Effect of digestive process on Maillard reaction indexes and antioxidant properties of breakfast cereals. Food Res Int 42:394–400
Angelino D, Cossu M, Marti A, Zanoletti M, Chiavaroli L, Brighenti F, Del Rio D, Martini D (2017) Bioaccessibility and bioavailability of phenolic compounds in bread: a review. Food Funct 8:2368–2393
Choi AS, Bae IY, Lee HG (2017) Predicting buckwheat flavonoids bioavailability in different food matrices under in vitro simulated human digestion. Cereal Chem 94:310–314
Świeca M (2016) Potentially bioaccessible phenolics, antioxidant activity and nutritional quality of young buckwheat sprouts affected by elicitation and elicitation supported by phenylpropanoid pathway precursor feeding. Food Chem 192:625–632
Delgado-Andrade C, Conde-Aguilera JA, Haro A, de la Cueva SP, Rufián-Henares JA (2010) A combined procedure to evaluate the global antioxidant response of bread. J Cereal Sci 52:239–246
Zieliński H, Ciesarová Z, Kukurová K, Zielińska D, Szawara-Nowak D, Starowicz M, Wronkowska M (2017) Effect of fermented and unfermented buckwheat flour on functional properties of gluten-free muffins. J Food Sci Technol 54:1425–1432
Szawara-Nowak D, Bączek N, Zieliński H (2016) Antioxidant capacity and bioaccessibility of buckwheat-enhanced wheat bread phenolics. J Food Sci Technol 53(1):621–630
Li F, Yuan Y, Yang X-I, Tao S, Ming J (2013) Phenolic profiles and antioxidant activity of buckwheat (Fagopyrum esculentum Moench and Fagopyrum tartaricum L. Gaerth) hulls, brans and flours. J Integr Agric 12:1684–1693
Jan U, Gani A, Ahmad M, Shah U, Baba WN, Masoodi FA, Maqsood S, Gani A, Wani IA, Wani SM (2015) Characterization of cookies made from wheat flour blended with buckwheat flour and effect on antioxidant properties. J Food Sci Technol 52:6334–6344
Chandrasekara A, Shahidi F (2012) Bioaccessibility and antioxidant potential of millet grain phenolics as affected by simulated in vitro digestion and microbial fermentation. J Funct Foods 4:226–237
Gawlik-Dziki U, Dziki D, Baraniak B, Lin R (2009) The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT-Food Sci Technol 42:137–143
Jacobs D-M, Gaudier E, van Duynhoven J, Vaughan E-E (2009) Non-digestible food ingredients, colonic microbiota and the impact on gut health and immunity: a role for metabolomics. Curr Drug Metab 10:41–54
Baublis AJ, Clydesdale FM, Decker EA (2000) Antioxidant in wheat-based breakfast cereals. Cereal Foods World 45:71–74
Marques MC, Hacke A, Camargo Neto CA, Mariutti LRB (2021) Impact of phenolic compounds in the digestion and absorption of carotenoids. Curr Opin Food Sci 39:190–196
Perez-Jimenez J, Saura-Calixto F (2005) Literature data may underestimate the actual antioxidant capacity of cereals. J Agric Food Chem 53:5036–5040
Gawlik-Dziki U, Durak A, Jamioł M, Świeca MI (2016) Interactions between antiradical and anti-inflammatory compounds from coffee and coconut affected by gastrointestinal digestion - in in vitro study. LWT Food Sci Technol 69:506–514
Hemery YM, Anson NM, Havenaar R, Haenen GRMM, Noort MWJ, Rouau X (2010) Dry-fractionation of wheat bran increases the bioaccessibility of phenolic acids in breads made from processed bran fractions. Food Res Int 43:1429–1438
Irakli M, Mygdalia A, Chatzopoulou P, Katsantonis D (2019) Impact of the combination of sourdough fermentation and hop extract addition on baking properties, antioxidant capacity and phenolics bioaccessibility of rice bran-enhanced bread. Food Chem 285:231–239
Acknowledgements
The study was financed from the research funds of the Institute of Animal Reproduction and Food Research of the Polish Academy of Science in Olsztyn, Poland. The study was supported by EU Project REFRESH (FP7-REGPOT-2010-1-264105).
Author information
Authors and Affiliations
Contributions
MW: conceived and designed the research program. NB: performed all the analyses. MW and CMH: wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Compliance with Ethics requirements
On behalf of all authors, the corresponding author states that this article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bączek, N., Haros, C.M. & Wronkowska, M. Buckwheat hull, a valuable bakery product ingredient: assessment of bioaccessible phenolics and antioxidant capacity. Eur Food Res Technol 249, 353–358 (2023). https://doi.org/10.1007/s00217-022-04120-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04120-w