Abstract
This study was aimed to produce pear cider (Perry), using small caliber pears cv Abate Fètel, fermented by Starmerella bacillaris and Saccharomyces cerevisiae in co-inoculated (COF) and sequential (SEF) mixed cultures in comparison with S. cerevisiae monoculture fermentation (AXF), evaluating the influence of yeast starter cultures on Perry characteristics. The perries were re-fermented in bottle by S. cerevisiae strain EC1118. During primary fermentation, growth and fermentation kinetics were different in the co-inoculated and sequential fermentations in comparison with pure S. cerevisiae fermentation; however, sugars were depleted, and 6% (v/v) ethanol was produced in all the trials. Glycerol content was significantly higher in mixed fermentations due to Starm. bacillaris metabolism (+ 20% in COF, and + 42% in SEF conditions). After re-fermentation in bottle, higher levels of 3-Methyl-1-butanol, 1-propanol, acetaldehyde and esters were detected in Perry from the mixed fermentations. All the Perries were accepted by the consumers (general liking values from 6.01 to 6.26). Perries’ appearance from mixed fermentations was described as less intense and more clear. The use of small caliber pears cv Abate Fètel and Starm. bacillaris in combination with S. cerevisiae in Perry production might be a suitable tool to obtain novel beverages with distinctive organoleptic features.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cider is a fermented juice of apple or pear (Perry) with ethanol concentration that does not exceed 8% (v/v) [1]. The production of this alcoholic fermented beverage is closely linked to the apples and pears producing countries, such as the United Kingdom, Ireland, France, Germany, Spain and the United States, which show also the highest consumption levels of these products [2, 3]. In Italy, the production of Perry is mainly restricted to the northern regions.
In the last few years, several pear producers recognized the possibility of Perry production as a tool for adding value to the raw material. This is the case of the Italian production of pears (Pyrus communis L.) cv Abate Fètel. The standard size for sale of this type of pear is 350/400 g per piece; hence, pears characterized by smaller size are wasted or unrewarding product on fresh commodities market. Considering that sustainable strategies in reducing surplus or waste fruit products are nowadays strongly recommended, this pear variety could be valorised and exploit as raw material in fermented beverages.
As concerns the microbiology of fermentation, most of the information in the scientific literature refers to the cider made with apples. As reported by Coton et al. [4], the cider can be obtained by spontaneous (French) or inoculated (British) fermentation. French cider production consists of three principal phases [4]: oxidative phase, alcoholic fermentation, and maturation phase. Each phase is characterized by different microbial species, at the beginning, slow fermentative yeasts, such as Metschnikowia, Hanseniaspora, and Candida genera, are the majority, successively microbial diversity largely decreases and the Saccharomyces uvarum becomes the dominant yeast. Finally, during the maturation phase, the population of S. uvarum decreases while sometimes other yeast species, such as Lachancea cidri, S. cerevisiae, Brettanomyces anomalus, or Brettanomyces bruxellensis, begin to grow. On the contrary, the British fermentation is much faster and more standardized as it is conducted using commercial starter yeasts (S. uvarum, Saccharomyces bayanus, or a mix of both). Unlike in Germany and Spain, the UK and France perform the carbonation of the cider that is normally saturated with ca. 5–6 g/L of CO2 (sparkling cider). Natural CO2 production (prise de mousse in French) is usually performed during the maturation phase. Indigenous yeasts or inoculated starter yeasts ferment residual or added sugars to form CO2 in bottles or in tanks. In view of the high microbial biodiversity of the natural processes, the use of only Saccharomyces starter strains to obtain a fermented beverage can limit the aromatic complexity of the final product. Indeed, co-culturing non-Saccharomyces yeasts with Saccharomyces cerevisiae could enrich the aromatic complexity of alcoholic beverages during cider brewing. However, there are few studies reporting the use of mixed cultures of Saccharomyces and non-Saccharomyces yeast species to carry out the cider or Perry fermentation [5, 6]. In particular, non-Saccharomyces yeast species, such as Hanseniaspora valbyensis, Williopsis saturnus and Wickerhamomyces anomalus (formerly Pichia anomala), Torulaspora delbrueckii, Hanseniaspora osmophila, Hanseniaspora vineae, Hanseniaspora uvarum, Starmerella bacillaris, and Zygosaccharomyces bailii, were studied, as single cultures or in co-inoculation with S. cerevisiae, for their capability to increase the flavor complexity of apple cider [7,8,9,10,11]. Nevertheless, few data are available regarding non-Saccharomyces yeast species effect on the sensorial characteristics of the Perry.
The main properties of Starm. bacillaris have been tested especially in wine and include the fructophilic character [12], the ability to produce high glycerol content [13], as well as terpenes, lactones [14] and organic acids [15], the ability to release mannoproteins [16], to modify anthocyanin and flavonoid profiles [17, 18] and finally to degrade malic acid [19]. Lorenzini et al. [9] tested the use of the single inoculum of Starm. bacillaris and other non-Saccharomyces species to ferment apple juice.The tested strain did not completely degrade the fermentable sugars, obtaining an ethanol concentration of 4.7% (v/v), lower compared to that of Torulaspora delbrueckii, Hanseniaspora osmophila, and Hanseniaspora uvarum, but it showed a higher content of the monoterpenes linalool and gerianol, suggesting an enhancement of the floral and fruit aroma of cider. To the best of our knowledge, no information is available in literature regarding the fermentation capacities of Starm. bacillaris in pear juice in monoculture or mixed fermentation, and its impact on the sensorial and chemical characteristics of the Perry.
Therefore, the aim of this study was to assess the effect of S. cerevisiae and Starm. bacillaris, when used in mixed fermentation, on the physicochemical and sensory properties of sparkling Perry obtained by small caliber pears.
Materials and methods
Pear juice and must preparation
Fresh, ripe, small size pears (Pyrus communis L.) cv Abate Fètel were supplied from the “Soc. Ag. Illuminati G.M.M. Snc” (Arezzo Italy) and stored at 20 °C. Pears were washed, chopped and shredded, 80 mg/L of potassium metabisulfite (K2S2O5, Enartis, Italy) was added to inhibit bacterial growth and prevent oxidation before the pressing. A stainless manual squeeze press (model Tommy, Palumbo Francesco s.r.l, Pomigliano d’Arco, Neaples, Italy) was used to press the pulp for 15 min. For clarification, the obtained juice was acidified to pH 4 with citric acid, 6 g/hL of EnartisZym RS (P) (Enartis, Italy) and 200 g/hL of Pluxbenton N (Enartis, Italy) were added according to manufactures instructions. Pear must was obtained after precipitation of solid particles after 72 h at 15 °C. Yields were calculated as the percentage of juice extracted from pears (wt/wt) and was 35%. Must composition was adjusted by adding nitrogen nutrients 0.625 g/L (Nutrientvit, Lallemand). The final pear must chemical and microbiological composition is reported in Table 1.
Yeast strains and culture conditions
The two strains used in this study were Starmerella bacillaris CB219, belonging to the collection of Department of Agricultural, Food and Forestry Systems (DAGRI) of the University of Florence (Italy) and isolated from spontaneous wine fermentations carried out at industrial scale in one Tuscan winery; and a commercial strain of Saccharomyces cerevisiae “Lalvin EC1118” (Lallemand Inc., Montreal, QC, Canada). Yeast strains were grown 24 h at 28 °C in 50 mL Erlenmeyer flasks with YEPD (Yeast Extract Peptone Dextrose) medium under stirring conditions (100 rpm). Yeast cell counts were performed by Neubauer improved counting chamber (Marienfeld, Lauda-Königshofe, Germany) and the viability by methylene blue staining. Before the inoculum, cells were recovered by centrifugation at 8000×g for 10 min, washed with physiological solution and resuspended in pear must to start the fermentation.
Preliminary laboratory-scale fermentations in pear must
The fermentations were carried out in 250 mL flasks containing 145 mL of pear must. Each fermentation was inoculated by the monoculture of Starm. bacillaris (coded SB) or by the monoculture of S. cerevisiae (coded SC) at a concentration of ca 106 cell/mL. The fermentations were carried out in duplicate at 20 °C and flasks were weighted once a day after gentle mixing (1 min) to monitor the fermentation progress (CO2 evolution) measuring the weight loss of the flasks until the end of the fermentation (constant weight for three consecutive days). For microbiological and chemical analyses, samples were daily collected.
Pilot scale fermentations and Perry production
The Perry production process is reported in Fig. 1. Stainless steel tanks (10 L of volume) containing 7 L of pear must were inoculated using as inoculum: S. cerevisiae EC1118 alone (axenic culture, AXF) at a concentration of ca 1 × 106 cells/mL; simultaneous co-inoculum of S. cerevisiae EC1118 (ca 1 × 104 cells/mL), and Starm. bacillaris CB219 strains (ca 1 × 106 cells/mL) (COF); sequential inoculum (SEF) of Starm. bacillaris CB219 (ca 5 × 106 cells/mL), followed, after 72 h of fermentation, by S. cerevisiae EC1118 (ca 5 × 106 cells/mL). The inoculated pear musts were periodically mixed. At the end of the alcoholic fermentation, pear ciders were cooled to 4 °C for 4 days for stabilization. Subsequently, they were bottled for the secondary fermentation. In this step, S. cerevisiae EC1118 was inoculated at concentration of 2 × 106 cells/mL and the following ingredients were added: 15 g/L sucrose, 0.4 g/L of Lalvin Nutrient Vit (Lallemand Inc.), and 40 mg/L of potassium metabisulfite. Finally, bottles were placed in an incubator at 20 °C for 60 days. Overpressure measurement of sparkling ciders was measured using an aphrometer (pressure gage) and was expressed in bars. Samples were collected daily for microbiological and chemical analyses. The final products were stored at 4 °C and subjected to chemical, microbiological and sensory analysis as described below. All fermentations were carried out in duplicate.
Microbiological analysis
Microbiological analyses of pear juice and pear sparkling ciders were performed. 1 mL of samples was homogenized with 9 mL of sterile saline solution. Serial dilutions were made and the diluted suspensions were plated on different culture media according to the microorganisms. Yeasts were quantified on WL Nutrient Agar medium (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) incubated 48 h at 30 °C in aerobic conditions, lactic acid bacteria on MRS Agar medium (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA), incubated 48 h at 30 °C in anaerobic conditions, and acetic bacteria on LF Agar medium (Glucose 10 g/L; Yeast extract 5 g/L; Pepton 5 g/L; Tomato Juice Broth 2 g/L) containing pimaricin (0.05 g/L) and penicillin (0.025 g/L), and incubated five days at 30 °C in aerobic conditions.
Molecular analysis
To investigate the dominance of the inoculated yeast strains during the primary fermentation of pear must in pilot-scale fermentations, a total of 25 isolates from each fermentation tank were characterized at strain level by molecular techniques and compared with the genomic profiles of the inoculated strains previously determined. In particular, the presumptive S. cerevisiae isolates were characterized by inter-δ PCR typing with δ12/δ21 primer pair (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) as reported by Legras and Karst [20]. The Starm. bacillaris isolates were subjected to Randomly amplified polymorphic DNA (RAPD) analysis using the primer M13 (50–GAGGGTGGCGGTTCT–30) [21] and the PCR protocol according to Reguant and Bordons [22]. All PCR reactions included both negative (DNA-free) and positive controls and were processed in an Applied Biosystems® 2720 Thermal Cycler (Life Technologies, Monza, Italy).
Chemical and analytical determination
The pH values were determined by a pH-meter (Metrohm Italiana Srl, Varese, Italy). Glucose, fructose, acetic acid, malic acid, lactic acid, glycerol, sorbitol and ethanol contents in pear juice and pear ciders, were determined by High-Pressure Liquid Chromatography (HPLC) according to Galli et al. [23]. Before injection, the samples were centrifuged 13,000 xg for 5 min. Separation was obtained with a Rezex ROA organic acid H + column (300 × 7.8 mm; Phenomenex, Castel Maggiore, Bologna, Italy), preceded by a security guard cartridge (carbo H 4 × 3.0 mm ID) connected to a refractive index detector (Varian, ProStar 350, Varian Inc, Palo Alto, CA, USA) and UV–VIS detector (λ = 210 nm) (ProStar 335, Varian Inc, Palo Alto, CA, USA). Elution was performed at 65 °C with 0.013 N H2SO4 eluent at flow rate of 0.6 mL/min. Data were collected and analyzed using the Galaxie software (Varian Inc, Palo Alto, CA, USA). Quantitative analysis was carried out by standard curves designed for each compound. All solvents were of HPLC quality, chemicals of analytical grade (99%). Total acidity, pH, sulfur dioxide were determined according to the Compendium of International Analysis of methods-OIV. Ammoniacal nitrogen, α-aminoacidic nitrogen, and l-malic acid were determined using enzymatic kits (Steroglass S.r.l. Perugia, Italy) following the manufacturer's instructions. Before the assays, pear juice and pear ciders were diluted ten-fold with distilled water.
Flavonols and flavan-3-ols on pear ciders were determined according to Hernández et al. [24]. Samples were concentrated to 50% of the initial volume under vacuum at 30 °C, and then extracted with ethyl acetate and diethyl ether. The combined organic fractions were evaporated to dryness and the residue, dissolved in methanol/water (80:20), was filtered (0.45 µm) and injected into the HPLC (Varian ProStar 210, Palo Alto, CA, USA), equipped with a diode array detector (DAD) and a reversed-phase column Chromsep Omnispher (5 μm particle, 250 × 4.6 mm; Varian, Palo Alto, CA, USA) preceded by a ChromSep guard column (10 × 3 mm i.d., Varian Palo Alto, CA, USA), thermostated at 25 °C. The mobile phase was (A) acetonitrile and (B) 2% (v/v) acetic acid in water; the gradient profile was 0–55 min, 100–80% B; 55–70 min, 80–50% B; 70–80 min, 50–5% B, followed by washing with acetonitrile and re-equilibration of the column from 110 to 125 min; the flow rate was 1 mL/min from the beginning to 60 min and 1.2 mL/min from this point to the end. Peak identification was confirmed with an HPLC–MS (Alliance 2695, Waters, USA), equipped with Photo Array Detector (2996, Waters, USA) and coupled to a triple quadrupole mass spectrometer (Quattro micro, Waters, USA) equipped with an electrospray ionization source (Z-spray, Waters, USA) using the same column, precolumn, eluents and gradient. Flavonols were detected by scanning from 210 to 600 nm and were quantified at 360 nm using quercetin, myricetin, kaempferol, quercetin-3-O-glucoside, quercetin-3-O-glucuronide, quercetin-3-O-galactoside, kaempferol-3-O-glucoside (Sigma-Aldrich, USA) myricetin-3-O-glucoside, myricetin-3-O-galactoside (from Extrasynthese, Cedex, France) as standards.. Flavan-3-ols were quantified at 280 nm using ( ±)-catechin and (−)-epicatechin (Sigma-Aldrich, USA) as standards.
Reduced (GSH) and Oxidized Glutathione (GSSG) of perries were determined according to Guerrini et al. [25], chromatographic separation was performed in a reverse phase column (Kinetex, 5 µm particle, 150 × 4.6 mm, 100 Å, Phenomenex Inc., Torrance, CA, USA) set at 25 °C and preceded by a Security Guard ULTRA guard cartridge (UHPLC C18, Phenomenex Inc., Torrance, CA, USA) The binary gradient was that described by Tuberoso et al. [26]. The quantitative analysis was performed using the external standard method; the stock standard solutions were prepared in 0.1 M HCl/Methanol (1:1, v/v) and stored at − 20 °C until use.
Higher alcohols (1-propanol, isobutanol, n-butanol, 2-Methyl-1-butanol, 3-Methyl-1-butanol), acetoin, diacetyl, acetaldehyde and ethyl acetate of pear ciders after the first and secondary fermentation were analyzed by gas chromatography equipped with glass column (6.6% CW 20 M BA 80/120 225, 2 m × 6 × 2 mm, Supelco Inc, Sigma-Aldrich, USA) as described by Romano et al.[27].
Consumer test
The three types of Perry (A = COF simultaneous co-inoculum of S. cerevisiae EC1118 and Starm. bacillaris CB219 strains; B = SEF sequential inoculum of Starm. bacillaris CB219 followed by S. cerevisiae EC1118; C = AXF axenic culture of S. cerevisiae EC1118) were evaluated by a panel of 85 consumers regular consumers of cider (self-reported) (55% women, age 18–40). Consumers participated in one evaluation session consisting of two sub-sessions: the first one for liking evaluation and the second one for describing perry sensory properties using a Check-All-That-Apply (CATA) methodology [28, 29]. Perry samples (30 mL) were presented in 100 mL white glasses at 13 °C identified by three-digit codes. Two independent sample sets, each consisting of the same three perry samples, were used for liking and CATA evaluations. The presentation order of the sample was randomized among assessors and sub-sessions using a balanced Latin square design. Assessors were asked to taste the samples and to express their liking on a 9-point category scale (1 = dislike extremely; 9 = extremely like) [30]. A product-specific CATA questionnaire to investigate the sensory characteristics of perry was developed based on one-on-one interviews with the EmoSemio approach [31] with a panel of fourteen consumers sharing comparable demographic characteristics with the main consumer panel. Interviews were conducted based on a modified version of the Repertory Grid technique and analyzed by applying a semiotic approach as described in Pierguidi et al. [32]. The developed list for CATA evaluation was composed of thirty-one descriptors of appearance (intense color, yellow, bright, clear, pale), aroma (odor by nose) (fruity, pear, fermented, yeast, floral, pungent, aromatic, persistent, intense), taste (sour, sweet, bitter, intense taste), flavor (pear, fermented fruit, delicate, complex, balanced, persistent) and mouthfeel (dry, full-bodied, smooth, fresh, sparkling, watery, drinkable). The order of attributes was randomized by sensory modality (appearance, aroma, taste/flavor and mouthfeel) across participants. Consumers were asked to describe sample appearance first, smell the sample and evaluate aroma, and then taste, flavor and mouthfeel after tasting the sample. After each sample, subjects rinsed their mouths with water for 60 s. Data were collected using a paper evaluation sheet.
Statistical and data analysis
The level of statistical significance was determined using one-way ANOVA (for multiple groups) followed by Tukey’s Test or Student’s t test (for comparisons between two groups) (GraphPad Prism 6 software package, San Diego, CA, USA). A p value of < 0.05 was considered to be significant. All analyses were conducted in triplicate and presented as average ± standard deviation. For the consumer test, a two-way ANOVA mixed model (factors: samples and consumers) with consumers as a random factor, was carried out to detect significant liking differences between the samples. Cochran Q-tests were performed on the CATA data to assess the differences between the frequency of attribute selection among samples. Post hoc pairwise comparisons were calculated using the Sheskin method with the level of significance set at 5%. XLSTAT, (version 2021.4.1, Addinsoft, NY, USA) was used for data analysis.
Results and discussion
Fermentation performances of Starm. bacillaris CB219 in pear juice
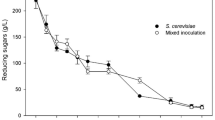
Starm. bacillaris CB219 was isolated as the dominant yeast strain from a spontaneous wine fermentation when the ethanol concentration was 6% (v/v). In some preliminary laboratory tests, this strain demonstrated good fermentation capabilities, high resistance to ethanol, and low acetic acid production in grape must (data not shown). To verify the maintenance of these fermentation performances during the Perry production, the CB219 strain was inoculated at a concentration of ca 1 × 106 CFU/mL in pear must. The performances of Starm. bacillaris CB219 strain were compared with those of the commercial strain S. cerevisiae EC1118 that is widely used as starter in the industrial productions of wine and cider (Fig. 2). Both strains reached a maximum population of 108 CFU/mL, however at different times, 5 days and 3 days for Starm. bacillaris and S. cerevisiae EC1118, respectively. As expected, due to its fructophilic character, Starm. bacillaris CB219 started to consume fructose immediately after inoculating, while glucose after 4–5 days. S. cerevisiae EC1118 degraded simultaneously glucose and fructose, reaching the same ethanol content of Starm. bacillaris CB219 even if in a shorter time. Both the strains completely exhausted the fermentable sugars, producing an ethanol concentration of 6% (v/v), however Starm. bacillaris CB219 in 13 days, whereas S. cerevisiae EC1118 in 5 days. Hence, Starm. bacillaris strain confirmed the results obtained in cider by Junior et al. [33] and proved to be much more efficient than the Starm. bacillaris strains tested by Lorenzini et al. [9] and Nadai et al. [34], which failed to complete alcoholic fermentation. As regards metabolites production, CB219 strain gradually produced glycerol until the 10th day, while EC1118 strain glycerol production stopped soon after the 3rd day. At the end of fermentation, the Perry obtained by CB219 strain was characterized by almost a double concentration of glycerol and acetic acid, and a minor content of malic acid compared to that of the Perry obtained by EC1118 strain (Table 2). The higher acetic acid content and malic acid degradation can be due to the contamination of acetic and lactic acid bacteria, observed only for the Perry SB (Table 2). The higher bacteria development is probably ascribable to the slower fermentation kinetic of Starm. bacillaris CB219 strain compared to that of S. cerevisiae EC1118 strain. As concerns sorbitol, no decrease was observed in either Perry. In general, pear and apple juice contain sorbitol that is considered unfermentable by yeasts [35]. This sugar alcohol has been proposed as an indicator for the authenticity of fruit juices and sweetness [35]. However, González Flores et al. [36, 37] identified two cryotolerant strains, belonging to Saccharomyces uvarum species, able to degrade sorbitol in pear juice. The worse fermentation performance and the highestbacterial contamination observed in the Perry inoculated with Starm. bacillaris CB219, indicated that its use as single culture is not suitable for Perry production; thus, it was not used for pilot-scale fermentation. Therefore, Starm. bacillaris CB219 was used in mixed culture with S. cerevisiae EC1118 strain for pilot-scale perry production.
Chemical and microbiological parameters (mean ± standard deviation) during the pear must fermentations carried out by inoculating the commercial starter S. cerevisiae EC1118 (a) and Starm. bacillaris CB219 (b). Cell counts of S. cerevisiae EC1118 ( ) and Starm. bacillaris CB219 (
) and Starm. bacillaris CB219 ( ) are plotted on the left axis; glucose (
) are plotted on the left axis; glucose ( ), fructose (
), fructose ( ), glycerol (
), glycerol ( ), and ethanol (
), and ethanol ( ) are plotted on the right axis
) are plotted on the right axis
Mixed fermentations by Starm. bacillaris CB219 and S. cerevisiae EC1118 in pear must (primary fermentation)
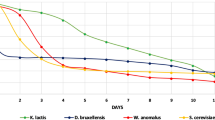
To evaluate the use of Starm. bacillaris in combination with S. cerevisiae for Perry production, at pilot-scale, two types of mixed cultures were tested: a simultaneous inoculum of S. cerevisiae EC1118 and Starm. bacillaris CB219 strains (COF), and sequential inoculum of these two strains (SEF). The sequential culture was obtained by first inoculating the strain Starm. bacillaris CB219 and after 72 h the strain S. cerevisiae EC1118. The latter was also inoculated in axenic culture as control (AXF). Figure 3 shows the trends of yeast populations and the evolution of the main substrates and yeast metabolites during the three tested conditions. The genotypic profiles obtained by inter-δ analysis for S. cerevisiae and RAPD-PCR by M13 primer for Starm. bacillaris were compared to those of the cultures used as inoculum; thus, they confirmed the dominance of the inoculated strains on indigenous yeasts until the end of the alcoholic fermentations. No significant differences were observed between COF and SEF as concern the kinetics of sugars degradation or of ethanol and glycerol release; indeed, both fermentations were completed in 5 days. On the contrary, in AXF condition, the alcoholic fermentation finished in only 3 days. The microbial and chemical composition of the Perries obtained from COF, SEF, and AXF is reported in Table 3. Ethanol content reached 6% (v/v) in all the fermented pear cider, whereas significantly higher glycerol and acetic acid concentration were detected in the SEF sample. Finally, AXF showed a higher amino acids amount than the other two experimental perries suggesting a possible different production of higher alcohols at the end of fermentation. Regarding the bacterial contaminations, acetic bacteria founded in COF and SEF might be due to the slower kinetic of fermentation conducted by the two mixed cultures compared to the axenic culture. In contrast, lactic acid bacteria concentrations were very low in all the tested fermentations. As regard to yeast concentrations, the trends were different based on the conditions. In the AXF inoculum, S. cerevisiae reached the stationary phase after 3 days, together with the sugar depletion. The maximum concentration was reached after the sixth day (1.8 × 108 CFU/mL), then it started decreasing. Also in the COF condition, both the yeast strains reached the stationary phase after 3 days (even if sugars were not depleted) and the maximum concentrations after 6 days. Starm. bacillaris remained in a higher concentration throughout the fermentation, reaching 1 × 108 CFU/mL, whereas S. cerevisiae EC1118 did not exceed 6 × 107 CFU/mL. Finally, in the sequential inoculum, both the yeast strains reached their stationary phase after 3 days, and unlike the COF conditions, both the strains reached ca 108 UFC/mL (1.06 × 108 CFU/mL and 1.22 × 108 CFU/mL, for S. cerevisiae and Starm. bacillaris, respectively).
Chemical and microbiological parameters (mean ± standard deviation) during the three Perry fermentations carried out by different yeast strains: A = simultaneous inoculum of S. cerevisiae EC1118 and Starm. bacillaris CB219 (COF); B = sequential inoculum of Starm. bacillaris CB219 and, after 72 h, S. cerevisiae EC1118 (SEF); C = axenic inoculum of S. cerevisiae EC1118 strain as control (AXF). Cell counts of Starm. bacillaris CB219 ( ) and S. cerevisiae EC1118 (
) and S. cerevisiae EC1118 ( ) are plotted on the left axis; glucose (
) are plotted on the left axis; glucose ( ), fructose (
), fructose ( ), glycerol (
), glycerol ( ), and ethanol (
), and ethanol ( ) are plotted on the right axis
) are plotted on the right axis
Secondary fermentations and chemical characterization of perries
To carry out the secondary fermentation, the perries were integrated with nitrogen source, sucrose and inoculated with 2 × 106 CFU/mL of S. cerevisiae EC1118. After these integrations, the ciders were bottled and incubated at 20 °C for 60 days. The release of CO2 during the time was monitored with aphrometers. Chemical and microbiological results are reported in Table S1. COF condition demonstrated a lower production of carbon dioxide gas than the other two ciders (4.1 atm versus 4.3 and 4.6 atm in SEF and AXF, respectively). Indeed, the concentration of S. cerevisiae EC1118 viable cells occurring in COF and SEF at the end of the secondary fermentation was almost 70% of the yeast population occurred in AXF (Table S1). The three Perry showed comparable fructose residue and a complete glucose consumption. Sorbitol amount was not different among the samples, being dependent on the variety and on the climatic conditions during the fruits growth rather than to the inoculum [35].
As potentially bioactive compound, due to its antioxidant properties, reduced glutathione (GSH) was determined in the experimental Perry. Indeed, in wine, reduced glutathione has been showed to limit the oxidation phenomena and protect aromatic substances. Table S1 shows the reduced and oxidized glutathione (GSSG) contents found in the three Perry after 60 days of bottle re-fermentation. No statistically significant difference was detected in the GSSG amount in the three Perry, whereas the highest content of GSH was found in the AXF condition followed by COF. Nevertheless, the total glutathione concentration was lower compared to the dose recommended by OIV resolutions [36,37,38] of 20 mg/L in wine to exert a protective effect against oxidation.
Phenolic composition of the three Perry was also evaluated (Table 4). Indeed, polyphenols, being of great interest for their natural antioxidant and health protective properties [39, 40], play important roles in the cider technological quality, although most of the available information are related only to apple cider. In cider, the polyphenols are associated to the color, bitterness and may be involved in the fermentative processes providing the cider aroma of astringency [41, 42]. Being inhibitors of the microbial growth, they can prevent the development of defects caused by lactic acid bacteria, such as acidification, mannitol taint, and excessive bitterness [41]. Finally, these compounds participate in the formation of sediments during the cider storage [43]. The phenolic composition and profile of cider are particularly affected by the fermenting microorganisms, the fruit variety, and the fruit ripening stage [44]. As regard to pear, this fruit mainly accumulates hydroxycinnamic acids, flavan-3-ols, procyanidins, flavonols (quercetin and isorhamnetin), even if the composition is highly cultivar-dependent [45]. Commisso et al. [46] analyzed 5 cultivar of pears and found out that Abate Fètel was characterized by a high antioxidant activity due to the presence of high levels procyanidins (catechin and epicatechin) and to hydroxycinnamic acids. To understand the impact of the three different types of fermentation on the phenolic composition of the three experimental Perry, analyses were performed after 60 days bottle re-fermentation. The three Perry polyphenolic composition was similar; however, some differences in gallic acid, p-coumaric acid, tyrosol, quercetin, and isorhamnetin concentration, were detected. AXF showed higher concentrations of p-coumaric acid and tyrosol, while lower concentrations of gallic acid, quercetin, and isorhamnetin than the other two perries. Tyrosol is a human health-promoting compound because of its antioxidant, anticarcinogenic, cardioprotective, and antimicrobial properties [47]. In wine, its production is significantly influenced either by the metabolic capabilities of the S. cerevisiae strains conducting the alcoholic fermentation [25], and by the fermentation rate. Indeed, slow fermentation kinetics lead to higher levels of hydroxytyrosol and tyrosol in must wine [48]. No information is available regarding the release of these two compounds during cider or Perry fermentation. p-Coumaric acid, together with ferulic and caffeic acids, are precursors of various volatile phenolic compounds of interest for cider [49], while gallic acid has antioxidant and anti-inflammatory potentials [50]. Quercetin and related flavonols, in addition to their antioxidant and inflammatory properties, exert vasodilator effects, protective effects under conditions of oxidative stress, effects of platelet antiaggregant, inhibition of LDL oxidation, reduction of adhesion molecules and other inflammatory markers and prevention of neuronal oxidative damage [51]. In view of these effects, the choice of the fermenting yeasts might affect and modulate the content of nutraceutical substances in Perry. As regards secondary yeast metabolites, higher alcohols were also assessed in the finished products. The GC analysis showed different concentration of ethyl acetate, 1-propanol, acetoin in Perry obtained with both yeasts species (SEF and COF) compared to that obtained with S. cerevisiae alone (AXF) (Table 5). COF Perry was characterized by a lower concentrations of 2- and 3-Methyl-1-butanol than the other two Perry, and of acetaldehyde if compared with AXF condition. Keeping in mind that higher alcohols are known to influence the organoleptic profile of cider [10] and that Starm. bacillaris proved to positively modulate cider volatile profile in the microfermentation trials as reported by Junior et al. [33], it was reasonable to assume that the three Perry had distinctive sensory characteristics. Hence, a sensory evaluation of the three Perry was performed.
Consumer test of the experimental Perry
No significant differences were found between samples for liking score (p = 0.24), pointing out that the alternative inoculum procedures did not affect consumer acceptance. All three samples showed mean liking scores between point 6 (“like slightly”) and point 7 (“like moderately”) of the 9-point hedonic scale (COF = 6.01; SEF = 6.31; AXF = 6.26), indicating that they were all well accepted by consumers. Conversely, the inoculum procedure affected the description of Perry's sensory profile. At the aggregate level, twenty-three over thirty-one terms showed an average frequency of use higher than 40% when consumers were asked to describe the sensory characteristics of the Perry using the CATA questionnaire. Attributes with a frequency of use below 40% were yeast, intense, pungent, and persistent aroma, bitter taste, and watery and full-bodied related to the mouthfeel. Sample description resulted in nine attributes in total significantly discriminating among samples (p < 0.05) for appearance (intense color and clear), aroma (pear, fermented, yeast, floral and aromatic) taste (bitter), and mouthfeel (sparkling) as reported in Fig. 4. When both S. cerevisiae and Starm. bacillaris strains were employed in Perry production, consumers described product appearance as less intense and more clear, in particular for the co-inoculum, as compared to the use of the axenic culture of S. cerevisiae only. This was probably due to the faster CO2 saturation of the AXF bottles, that protected Perry from oxidation caused by the periodic mixing. The terms aromatic and pear were less used to describe the aroma of SEF Perry as compared to the other samples while the opposite was found for fermented. Perry obtained by non-sequential inoculum (both co-inoculum and S. cerevisiae alone) was described as more aromatic and more connoted by pear odor. Furthermore, the use of sequential inoculum of Starm. bacillaris and S. cerevisiae resulted in perry being described as more connoted by yeast and less by floral aroma as compared to the simultaneous co-inoculum of the same strains. Differences in inoculum sequence affected also the perception of Perry’s taste with the one obtained by simultaneous co-inoculum being described as more bitter as compared to sequential inoculum, that showed a high content of glycerol, probably responsible for a sweeter taste. Finally, simultaneous co-inoculum resulted in a lower sparkling mouthfeel as compared to the other procedures.
Percentage of participant selection for the attributes that significantly discriminated among samples in the CATA questionnaire (p < 0.05). Different letters indicate significant differences (p < 0.05) according to Sheskin post hoc test. COF (light gray bars): Perry obtained by simultaneous inoculum of S. cerevisiae EC1118 and Starm. bacillaris CB219 strains; SEF (white bars): Perry obtained by sequential inoculum of Starm. bacillaris CB219 and, after 72 h, S. cerevisiae EC1118; AXF (dark gray bars): Perry obtained by axenic inoculum of S. cerevisiae EC1118 strain
Conclusion
In this study, the effect of a mixed fermentation using S. cerevisiae EC 1118 and Starm. bacillaris CB219 on the chemical and sensory properties of sparkling pear fermented beverage (Perry) was evaluated. The analyses of the experimental Perry indicated that different inoculum led to peculiar characteristics of the final product, which were well accepted by the consumers and possessed adequate technological features. In conclusion, this preliminary evaluation indicated that the use of Starm. bacillaris together with S. cerevisiae is a suitable tool to obtain novel low alcoholic beverages with distinctive organoleptic features meeting the increasing consumer demand for this type of product. Moreover by valorizing an unrewarding product such as low size pears, the Perry production might contribute to the reduction of fruit waste and to the circular economy.
References
Jarvis B (1996) Cider, perry, fruit wines and other alcoholic fruit beverages. In: Arthey D, Ashurst PR (eds) Fruit processing. Springer, Boston, pp 97–134
Joshi VK, Sharma S, Thakur AD (2017) Wines: white, red, sparkling, fortified, and cider. In: Pandey A, Sanromán MA, Du G, Soccol CR, Dussap CG (eds) Current developments in biotechnology and bioengineering. Elsevier, Amsterdam, pp 353–406
Merwin IA, Valois S, Padilla-Zakour OI (2008) Cider apples and cider-making techniques in Europe and North America. In: Janick J (ed) Horticultural reviews (vol 34), John Wiley & Sons. Hoboken, NJ, USA, pp 365–416
Coton E, Coton M, Guichard H (2016) Cider (Cyder; Hard Cider): The product and its manufacture. In: Caballero B, Finglas PM, Toldrà F (eds), Encyclopedia of food and health. Academic Press, Oxford, pp. 119–128.
Hou CY, Huang PH, Lai YT, Lin SP, Liou BK, Lin HW, Hsieh CW, Cheng KC (2022) Screening and identification of yeasts from fruits and their coculture for cider production. Fermentation 8(1):1. https://doi.org/10.3390/fermentation8010001
Ye M, Yue T, Yuan Y (2014) Effects of sequential mixed cultures of Wickerhamomyces anomalus and Saccharomyces cerevisiae on apple cider fermentation. FEMS Yeast Res 14(6):873–882. https://doi.org/10.1111/1567-1364.12175
Aung MT, Lee PR, Yu B, Liu SQ (2015) Cider fermentation with three Williopsis saturnus yeast strains and volatile changes. Ann Microbiol 65(2):921–928. https://doi.org/10.1007/s13213-014-0935-7
Liu SQ, Aung MT, Lee PR, Yu B (2016) Yeast and volatile evolution in cider co-fermentation with Saccharomyces cerevisiae and Williopsis saturnus. Ann Microbiol 66(1):307–315. https://doi.org/10.1007/s13213-015-1110-5
Lorenzini M, Simonato B, Slaghenaufi D, Ugliano M, Zapparoli G (2019) Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT-Food Sci Technol 99:224–230. https://doi.org/10.1016/j.lwt.2018.09.075
Xu Y, Zhao GA, Wang LP (2006) Controlled formation of volatile components in cider making using a combination of Saccharomyces cerevisiae and Hanseniaspora valbyensis yeast species. J Ind Microbiol Biotech 33(3):192–196. https://doi.org/10.1007/s10295-005-0051-6
Ye M, Yue T, Yuan Y (2014) Changes in the profile of volatile compounds and amino acids during cider fermentation using dessert variety of apples. Eur Food Res Technol 239(1):67–77. https://doi.org/10.1007/s00217-014-2204-1
Englezos V, Rantsiou K, Torchio F, Rolle L, Gerbi V, Cocolin L (2015) Exploitation of the non-Saccharomyces yeast Starmerella bacillaris (synonym Candida zemplinina) in wine fermentation: physiological and molecular characterizations. Int J Food Microbiol 199:33–40. https://doi.org/10.1016/j.ijfoodmicro.2015.01.009
Zara G, Mannazzu I, Del Caro A, Budroni M, Pinna MB, Murru M, Farris GA, Zara S (2014) Wine quality improvement through the combined utilisation of yeast hulls and Candida zemplinina/Saccharomyces cerevisiae mixed starter cultures. Aust J Grape Wine Res 20(2):199–207. https://doi.org/10.1111/ajgw.12078
Sadoudi M, Tourdot-Maréchal R, Rousseaux S, Steyer D, Gallardo-Chacón JJ, Ballester J, Vichi S, Guérin-Schneider R, Caixach J, Alexandre H (2012) Yeast–yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol 32(2):243–253. https://doi.org/10.1016/j.fm.2012.06.006
Magyar I, Nyitrai-Sárdy D, Leskó A, Pomázi A, Kállay M (2014) Anaerobic organic acid metabolism of Candida zemplinina in comparison with Saccharomyces wine yeasts. Int J Food Microbiol 178:1–6. https://doi.org/10.1016/j.ijfoodmicro.2014.03.002
Domizio P, Liu Y, Bisson LF, Barile D (2014) Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol 43:5–15. https://doi.org/10.1016/j.fm.2014.04.005
Mangani S, Buscioni G, Collina L, Bocci E, Vincenzini M (2011) Effects of microbial populations on anthocyanin profile of Sangiovese wines produced in Tuscany. Italy Am J Enol Vitic 62(4):487–494. https://doi.org/10.5344/ajev.2011.11047
Mangani S, Buscioni G, Guerrini S, Granchi L (2020) Influence of sequential inoculum of Starmerella bacillaris and Saccharomyces cerevisiae on flavonoid composition of monovarietal Sangiovese wines. Yeast 37(9–10):549–557. https://doi.org/10.1002/yea.3474
Tofalo R, Schirone M, Torriani S, Rantsiou K, Cocolin L, Perpetuini G, Suzzi G (2012) Diversity of Candida zemplinina strains from grapes and Italian wines. Food Microbiol 29(1):18–26. https://doi.org/10.1016/j.fm.2011.08.014
Legras JL, Karst F (2003) Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterisation. FEMS Microbiol Lett 221:249–255. https://doi.org/10.1016/S0378-1097(03)00205-2
Huey BING, Hall JEFF (1989) Hypervariable DNA fingerprinting in Escherichia coli: minisatellite probe from bacteriophage M13. J Bacteriol 171(5):2528–2532. https://doi.org/10.1128/jb.171.5.2528-2532.1989
Reguant C, Bordons A (2003) Typification of Oenococcus oeni strains by multiplex RAPD-PCR and study of population dynamics during malolactic fermentation. J Appl Microbiol 95(2):344–353. https://doi.org/10.1046/j.1365-2672.2003.01985.x
Galli V, Venturi M, Cardone G, Pini N, Marti A, Granchi L (2021) In situ dextran synthesis by Weissella confusa Ck15 and Leuconostoc pseudomesenteroides DSM 20193 and their effect on chickpea sourdough bread. Int J Food Sci 56(10):5277–5285. https://doi.org/10.1111/ijfs.15097
Hernández T, Estrella I, Carlavilla D, Martín-Álvarez PJ, Moreno-Arribas MV (2006) Phenolic compounds in red wine subjected to industrial malolactic fermentation and ageing on lees. Anal Chim Acta 563(1–2):116–125. https://doi.org/10.1016/j.aca.2005.10.061
Guerrini S, Mangani S, Romboli Y, Luti S, Pazzagli L, Granchi L (2018) Impact of Saccharomyces cerevisiae strains on health-promoting compounds in wine. Fermentation 4(2):26. https://doi.org/10.3390/fermentation4020026
Tuberoso CIG, Congiu F, Serreli G, Mameli S (2015) Determination of dansylated amino acids and biogenic amines in Cannonau and Vermentino wines by HPLC-FLD. Food Chem 175:29–35. https://doi.org/10.1016/j.foodchem.2014.11.120
Romano P, Marchese R, Laurita C, Saleano G, Turbanti L (1999) Biotechnological suitability of Saccharomycodes ludwigii for fermented beverages. World J Microbiol Biotechno 15(4):451–454. https://doi.org/10.1023/A:1008948623024
Adams J, Williams A, Lancaster B, Foley M (2007) Advantages and uses of check-all-that-apply response compared to traditional scaling of attributes for salty snacks. In 7th Pangborn sensory science symposium (Vol. 16). Minneapolis, USA, 12–16 August, 2007
Meyners M, Castura JC (2014) Check-all-that-apply questions. In: Varela P, Ares G (eds) Novel techniques in sensory characterization and consumer profiling. CRC Press, Boca Raton, pp 271–305
Peryam DR, Pilgrim FJ (1957) Hedonic scale method of measuring food preferences. Food Technol 11(9):9–14
Spinelli S, Masi C, Dinnella C, Zoboli GP, Monteleone E (2014) How does it make you feel? A new approach to measuring emotions in food product experience. Food Qual Prefer 37:109–122. https://doi.org/10.1016/j.foodqual.2013.11.009
Pierguidi L, Spinelli S, Dinnella C, Prescott J, Monteleone E (2019) Individual differences in perceived complexity are associated with different affective responses to alcoholic cocktails. Food Qual Prefer 76:47–59. https://doi.org/10.1016/j.foodqual.2019.03.010
Junior WJL, Binati RL, Felis GE, Slaghenaufi D, Ugliano M, Torriani S (2020) Volatile organic compounds from Starmerella bacillaris to control gray mold on apples and modulate cider aroma profile. Food Microbiol 89:103446. https://doi.org/10.1016/j.fm.2020.103446
Nadai C, Fernandes Lemos WJ, Favaron F, Giacomini A, Corich V (2018) Biocontrol activity of Starmerella bacillaris yeast against blue mold disease on apple fruit and its effect on cider fermentation. PLoS ONE 13(9):e0204350. https://doi.org/10.1371/journal.pone.0204350
Dietrich H, Krüger-Steden E, Patz CD, Will F, Rheinberger A, Hopf I (2007) Increase of sorbitol in pear and apple juice by water stress, a consequence of climatic change. Fruit Proc 6:348–355
González Flores M, Origone AC, Bajda L, Rodríguez ME, Lopes CA (2021) Evaluation of cryotolerant yeasts for the elaboration of a fermented pear beverage in Patagonia: Physicochemical and sensory attributes. Int J Food Microbiol 345:109129. https://doi.org/10.1016/j.ijfoodmicro.2021.109129
RESOLUTION OIV-OENO 445-2015. Treatment of Must with Glutathione. http://www.oiv.int/public/medias/1686/oiv-oeno-445-2015-en.pdf. Accessed 27 May 2020
RESOLUTION OIV-OENO 446-2015. Treatment of Wine with Glutathione. Available online: http://www.oiv.int/public/medias/1687/oiv-oeno-446-2015-en.pdf. Accessed 27 May 2020
Tsao R, Yang R, Xie S, Sockovie E, Khanizadeh S (2005) Which polyphenolic compounds contribute to the total antioxidant activities of apple? J Agric Food Chem 53(12):4989–4995. https://doi.org/10.1021/jf048289h
Vanzani P, Rossetto M, Rigo A, Vrhovsek U, Mattivi F, D’Amato E, Scarpa M (2005) Major phytochemicals in apple cultivars: contribution to peroxyl radical trapping efficiency. J Agric Food Chem 53(9):3377–3382. https://doi.org/10.1021/jf040482o
Alonso-Salces RM, Barranco A, Abad B, Berrueta LA, Gallo B, Vicente F (2004) Polyphenolic profiles of Basque cider apple cultivars and their technological properties. J Agric Food Chem 52(10):2938–2952. https://doi.org/10.1021/jf0354161
Lea AG, Drilleau JF (2003) Cidermaking. In: Lea AGH, Piggott JR (eds) Fermented beverage production. Springer, Boston, pp 59–87
Tarko T, Duda-Chodak A, Sroka P, Januszek M (2020) Effect of musts oxygenation at various stages of cider production on oenological parameters, antioxidant activity, and profile of volatile cider compounds. Biomolecules 10(6):890. https://doi.org/10.3390/biom10060890
Zhang S, Hu C, Guo Y, Wang X, Meng Y (2021) Polyphenols in fermented apple juice: beneficial effects on human health. J Funct Foods 76:104294. https://doi.org/10.1016/j.jff.2020.104294
Brahem M, Renard CM, Eder S, Loonis M, Ouni R, Mars M, Le Bourvellec C (2017) Characterization and quantification of fruit phenolic compounds of European and Tunisian pear cultivars. Food Res Int 95:125–133. https://doi.org/10.1016/j.foodres.2017.03.002
Commisso M, Bianconi M, Poletti S, Negri S, Munari F, Ceoldo S, Guzzo F (2021) Metabolomic profiling and antioxidant activity of fruits representing diverse apple and pear cultivars. Biology 10(5):380. https://doi.org/10.3390/biology10050380
Karković Marković A, Torić J, Barbarić M, Jakobušić Brala C (2019) Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 24(10):2001. https://doi.org/10.3390/molecules24102001
Romboli Y, Mangani S, Buscioni G, Granchi L, Vincenzini M (2015) Effect of Saccharomyces cerevisiae and Candida zemplinina on quercetin, vitisin A and hydroxytyrosol contents in Sangiovese wines. World J Microbiol Biotechnol 31(7):1137–1145. https://doi.org/10.1007/s11274-015-1863-9
Buron N, Guichard H, Coton E, Ledauphin J, Barillier D (2011) Evidence of 4-ethylcatechol as one of the main phenolic off-flavour markers in French ciders. Food Chem 125(2):542–548. https://doi.org/10.1016/j.foodchem.2010.09.046
Kahkeshani N, Farzaei F, Fotouhi M, Alavi SS, Bahramsoltani R, Naseri R, Momtaz S, Abbasabadi Z, Rahimi R, Farzaei MH, Bishayee A (2019) Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J Basic Med Sci 22(3):225. https://doi.org/10.22038/ijbms.2019.32806.7897
Wang Y, Zhang ZZ, Wu Y, Ke JJ, He XH, Wang YL (2013) Quercetin postconditioning attenuates myocardial ischemia/reperfusion injury in rats through the PI3K/Akt pathway. Braz J Med Biol Res 46:861–867. https://doi.org/10.1590/1414-431X20133036
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conceptualization: SG, DB, GF; formal analysis: SG, VG, DB, GF, SM, LP; writing—original draft preparation: SG, VG; Investigation: DB, GF, SM, LP; writing—review & Editing: VG, LP, LG; Supervision: LG.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Compliance with ethics requirements
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guerrini, S., Galli, V., Barbato, D. et al. Effects of Saccharomyces cerevisiae and Starmerella bacillaris on the physicochemical and sensory characteristics of sparkling pear cider (Perry). Eur Food Res Technol 249, 341–352 (2023). https://doi.org/10.1007/s00217-022-04119-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04119-3