Abstract
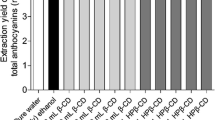
The objective of this study was the production of green extracts from Cornelian cherry pomace and the valorization of their anthocyanin content for their potential use as food colorants. Aqueous solutions of β-cyclodextrin were used both as green extraction solvents as well as means of anthocyanins’ stabilization. The extracts were analyzed by a novel liquid chromatographic method coupled to triple quadrupole time-of-flight mass spectrometry analytical methodology (LC-QTOF-MS) to assess their anthocyanin content. FTIR spectroscopy was used to investigate possible interactions between cyclodextrin and Cornelian cherry’s anthocyanins. The stability of anthocyanins was evaluated under different pH conditions and in the presence of metal ion Fe+2. The extracts were finally evaluated as colorants in two different food systems. The results showed that the color of β-cyclodextrin extract was preserved through storage. Additionally, the combination of β-cyclodextrin extraction and storage at low temperature contributes to the development of a stable acidic non-carbonated red beverage.





Similar content being viewed by others
References
Szczepaniak OM, Kobus-Cisowska J, Kusek W, Przeor M (2019) Functional properties of Cornelian cherry (Cornus mas L.): a comprehensive review. Eur Food Res Technol 245:2071–2087. https://doi.org/10.1007/s00217-019-03313-0
Pantelidis GE, Vasilakakis M, Manganaris GA, Diamantidis G (2007) Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem 102:777–783. https://doi.org/10.1016/j.foodchem.2006.06.021
Yilmaz K, Ercisli S, Zengin Y et al (2009) Preliminary characterisation of cornelian cherry (Cornus mas L.) genotypes for their physico-chemical properties. Food Chem 114:408–412. https://doi.org/10.1016/j.foodchem.2008.09.055
Koley TK, Singh S, Khemariya P et al (2014) Evaluation of bioactive properties of Indian carrot (Daucus carota L.): a chemometric approach. Food Res Int 60:76–85. https://doi.org/10.1016/j.foodres.2013.12.006
Ahmadiani N, Robbins RJ, Collins TM, Giusti MM (2014) Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. J Agric Food Chem 62:7524–7531. https://doi.org/10.1021/jf501991q
Moldovan B, David L (2014) Influence of temperature and preserving agents on the stability of cornelian cherries anthocyanins. Molecules 19:8177–8188. https://doi.org/10.3390/molecules19068177
Dumitraşcu L, Stănciuc N, Borda D et al (2021) Microencapsulation of bioactive compounds from cornelian cherry fruits using different biopolymers with soy proteins. Food Biosci. https://doi.org/10.1016/j.fbio.2021.101032
Chatham LA, Howard JE, Juvik JA (2020) A natural colorant system from corn: flavone-anthocyanin copigmentation for altered hues and improved shelf life. Food Chem 310:125734. https://doi.org/10.1016/j.foodchem.2019.125734
Chung C, Rojanasasithara T, Mutilangi W, McClements DJ (2016) Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem 212:596–603. https://doi.org/10.1016/j.foodchem.2016.06.025
Trouillas P, Sancho-García JC, De Freitas V et al (2016) Stabilizing and modulating color by copigmentation: insights from theory and experiment. Chem Rev 116:4937–4982. https://doi.org/10.1021/acs.chemrev.5b00507
Gençdağ E, Özdemir EE, Demirci K et al (2022) Copigmentation and stabilization of anthocyanins using organic molecules and encapsulation techniques. Curr Plant Biol. https://doi.org/10.1016/j.cpb.2022.100238
Chung C, Rojanasasithara T, Mutilangi W, McClements DJ (2015) Enhanced stability of anthocyanin-based color in model beverage systems through whey protein isolate complexation. Food Res Int 76:761–768. https://doi.org/10.1016/j.foodres.2015.07.003
Tutunchi P, Roufegarinejad L, Hamishehkar H, Alizadeh A (2019) Extraction of red beet extract with β-cyclodextrin-enhanced ultrasound assisted extraction: a strategy for enhancing the extraction efficacy of bioactive compounds and their stability in food models. Food Chem 297:124994. https://doi.org/10.1016/j.foodchem.2019.124994
Cai R, Yuan Y, Cui L et al (2018) Cyclodextrin-assisted extraction of phenolic compounds: current research and future prospects. Trends Food Sci Technol 79:19–27. https://doi.org/10.1016/j.tifs.2018.06.015
Howard LR, Brownmiller C, Prior RL, Mauromoustakos A (2013) Improved stability of chokeberry juice anthocyanins by β-cyclodextrin addition and refrigeration. J Agric Food Chem 61:693–699. https://doi.org/10.1021/jf3038314
Quan W, He W, Qie X et al (2020) Effects of β-cyclodextrin, whey protein, and soy protein on the thermal and storage stability of anthocyanins obtained from purple-fleshed sweet potatoes. Food Chem 320:126655. https://doi.org/10.1016/j.foodchem.2020.126655
Kritikou E, Kalogiouri NP, Kolyvira L, Thomaidis NS (2020) Target and suspect HRMS metabolomics for the 13 varieties of olive leaves and drupes from Greece. Molecules. https://doi.org/10.3390/molecules25214889
Martins N, Roriz CL, Morales P et al (2016) Food colorants: challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci Technol 52:1–15. https://doi.org/10.1016/j.tifs.2016.03.009
Mourtzinos I, Prodromidis P, Grigorakis S et al (2018) Natural food colorants derived from onion wastes: application in a yoghurt product. Electrophoresis 39:1975–1983. https://doi.org/10.1002/elps.201800073
Pinasseau L, Vallverdú-Queralt A, Verbaere A et al (2017) Cultivar diversity of grape skin polyphenol composition and changes in response to drought investigated by LC-MS based metabolomics. Front Plant Sci 8:1–24. https://doi.org/10.3389/fpls.2017.01826
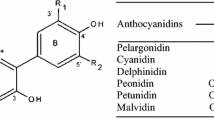
Kucharska AZ, Szumny A, Sokól-Letowska A et al (2015) Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J Food Compos Anal 40:95–102. https://doi.org/10.1016/j.jfca.2014.12.016
Eeram NAPS, Chutzki ROS, Handra AMC, Air MUGN (2002) Anthocyanins in Cornus species. J Agric Food Chem 50:2519–2523
Giusti MM, Wrolstad RE (2005) Characterization and measurement of anthocyanins by UV-visible spectroscopy. Handb Food Anal Chem 2–2:19–31. https://doi.org/10.1002/0471709085.ch18
Patras A, Brunton NP, O’Donnell C, Tiwari BK (2010) Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci Technol 21:3–11. https://doi.org/10.1016/j.tifs.2009.07.004
Aguilera Y, Mojica L, Rebollo-Hernanz M et al (2016) Black bean coats: new source of anthocyanins stabilized by β-cyclodextrin copigmentation in a sport beverage. Food Chem 212:561–570. https://doi.org/10.1016/j.foodchem.2016.06.022
Horai H, Arita M, Kanaya S et al (2010) MassBank: a public repository for sharing mass spectral data for life sciences. J Mass Spectrom 45:703–714. https://doi.org/10.1002/jms.1777
Homoki JR, Nemes A, Fazekas E et al (2016) Anthocyanin composition, antioxidant efficiency, and α-amylase inhibitor activity of different Hungarian sour cherry varieties (Prunus cerasus L.). Food Chem 194:222–229. https://doi.org/10.1016/j.foodchem.2015.07.130
Popović BM, Blagojević B, Latković D et al (2021) A one step enhanced extraction and encapsulation system of cornelian cherry (Cornus mas L.) polyphenols and iridoids with β-cyclodextrin. LWT. https://doi.org/10.1016/j.lwt.2021.110884
Rakić V, Rinnan Å, Polak T et al (2019) pH-induced structural forms of cyanidin and cyanidin 3-O-β-glucopyranoside. Dye Pigment 165:71–80. https://doi.org/10.1016/J.DYEPIG.2019.02.012
Tang P, Monica Giusti M (2020) Metal chelates of petunidin derivatives exhibit enhanced color and stability. Foods 9:11–15. https://doi.org/10.3390/foods9101426
Andrés-Bello A, Barreto-Palacios V, García-Segovia P et al (2013) Effect of pH on color and texture of food products. Food Eng Rev 5:158–170. https://doi.org/10.1007/s12393-013-9067-2
Fernandes A, Sousa A, Azevedo J et al (2013) Effect of cyclodextrins on the thermodynamic and kinetic properties of cyanidin-3-O-glucoside. Food Res Int 51:748–755. https://doi.org/10.1016/j.foodres.2013.01.037
Li X-D, Li J, Wang M, Jiang H (2016) Copigmentation effects and thermal degradation kinetics of purple sweet potato anthocyanins with metal ions and sugars. Appl Biol Chem 59:15–24. https://doi.org/10.1007/s13765-015-0140-9
Sigurdson GT, Robbins RJ, Collins TM, Giusti MM (2016) Evaluating the role of metal ions in the bathochromic and hyperchromic responses of cyanidin derivatives in acidic and alkaline pH. Food Chem 208:26–34. https://doi.org/10.1016/j.foodchem.2016.03.109
Molaeafard S, Jamei R, Poursattar Marjani A (2021) Co-pigmentation of anthocyanins extracted from sour cherry (Prunus cerasus L.) with some organic acids: Color intensity, thermal stability, and thermodynamic parameters. Food Chem 339:128070. https://doi.org/10.1016/j.foodchem.2020.128070
Rostamizadeh E, Iranbakhsh A, Majd A et al (2020) Green synthesis of Fe2O3 nanoparticles using fruit extract of Cornus mas L. and its growth-promoting roles in Barley. J Nanostructure Chem 10:125–130. https://doi.org/10.1007/s40097-020-00335-z
Ahmad M, Ashraf B, Gani A, Gani A (2018) Microencapsulation of saffron anthocyanins using β glucan and β cyclodextrin: Microcapsule characterization, release behaviour & antioxidant potential during in-vitro digestion. Int J Biol Macromol 109:435–442. https://doi.org/10.1016/j.ijbiomac.2017.11.122
Kalantari S, Roufegarinejad L, Pirsa S et al (2021) β-Cyclodextrin-assisted extraction of phenolic compounds from pomegranate (Punica granatum L) peel: a new strategy for anthocyanin copigmentation. Lwt 151:112136. https://doi.org/10.1016/j.lwt.2021.112136
Sigurdson GT, Robbins RJ, Collins TM, Giusti MM (2017) Spectral and colorimetric characteristics of metal chelates of acylated cyanidin derivatives. Food Chem 221:1088–1095. https://doi.org/10.1016/j.foodchem.2016.11.052
Patras A (2019) Stability and colour evaluation of red cabbage waste hydroethanolic extract in presence of different food additives or ingredients. Food Chem 275:539–548. https://doi.org/10.1016/j.foodchem.2018.09.100
Guan Y, Zhong Q (2015) The improved thermal stability of anthocyanins at pH 5.0 by gum arabic. LWT Food Sci Technol 64:706–712. https://doi.org/10.1016/j.lwt.2015.06.018
Tachibana N, Kimura Y, Ohno T (2014) Examination of molecular mechanism for the enhanced thermal stability of anthocyanins by metal cations and polysaccharides. Food Chem 143:452–458. https://doi.org/10.1016/j.foodchem.2013.08.017
Carpenter R, O’Grady MN, O’Callaghan YC et al (2007) Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci 76:604–610. https://doi.org/10.1016/j.meatsci.2007.01.021
Garrido MD, Auqui M, Martí N, Linares MB (2011) Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT Food Sci Technol 44:2238–2243. https://doi.org/10.1016/j.lwt.2011.07.003
Prommachart R, Belem TS, Uriyapongson S et al (2020) The effect of black rice water extract on surface color, lipid oxidation, microbial growth, and antioxidant activity of beef patties during chilled storage. Meat Sci 164:108091. https://doi.org/10.1016/j.meatsci.2020.108091
Acknowledgements
Authors would like to thank Physis Ingredients (Serres, Greece) for providing the Cornelian cherry pomace as well as Interdisciplinary Agri‐Food Center (KEAGRO), Aristotle University of Thessaloniki for providing access to the equipment of the unit.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AL: writing—original draft, methodology, investigation. SC: writing—review and editing, formal analysis. NPK: methodology, investigation, writing—review and editing. UMS: writing—review and editing IM: conceptualization, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interests
There are no conflicts of interest.
Human and animal rights
The Research did not involve Human Participants and/or Animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loukri, A., Christaki, S., Kalogiouri, N.P. et al. Anthocyanin-rich extracts from Cornelian cherry pomace as a natural food colorant: a spectroscopic and LC-QTOF-MS study. Eur Food Res Technol 248, 2901–2912 (2022). https://doi.org/10.1007/s00217-022-04099-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04099-4