Abstract
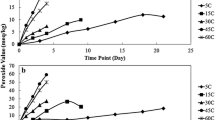
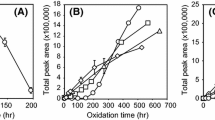
The mechanisms of volatile species formation for hydroperoxylated metabolites of unsaturated fatty acids in grass carp oil were investigated. All oil samples were heated at 110 °C for 8 various durations. The hydroperoxylated metabolites were evaluated by ultra-performance liquid chromatography coupled with time-of-flight mass spectrometry, solid-phase microextraction gas chromatography mass spectrometry and conventional chemical indicators. Compared to fresh fish oil, the content of monounsaturated fatty acids and saturated fatty acids with higher oxidation stability in the heating samples was significantly increased (P < 0.05), while the content of polyunsaturated fatty acids was significantly reduced (P < 0.05). A total of 35 triglycerides were determined, of these, the relative content of carbon numbers (CNs) 54, 56 and 58 was dramatically decreased, while CN50 and CN52 were gradually increased. Partial least-squares discriminant analysis indicated that continuous heating had different effects on volatile substances and twelve volatile species variables associated with lipid oxidation from the eight various heating periods were identified. In addition, sixteen oxidized metabolites were identified and their content was significantly increased (P < 0.05), except the 13-HODE, 9-HOTrE, 5-HETE and 12-HETE. The metabolic pathways of grass carp oil under continuous heating were clarified based on critical volatile components and oxidized metabolites, intending to reveal the oxidation paradigm of oil exposed to high temperatures.



Similar content being viewed by others
Abbreviations
- UPLC-TOF–MS/MS:
-
Ultra-performance liquid chromatography-time-of-flight mass spectrometry
- SPME–GC–MS:
-
Solid-phase microextraction gas chromatography mass spectrometry
- MUFAs:
-
Monounsaturated fatty acids
- SFAs:
-
Saturated fatty acids
- PUFAs:
-
Polyunsaturated fatty acids
- TAGs:
-
Triglycerides
- CNs:
-
Carbon numbers
- PLS-DA:
-
Partial least-squares discriminant analysis
- OA:
-
Oleic acid
- LA:
-
Linoleic acid
- ALA:
-
Linolenic acid
- ARA:
-
Arachidonic acid
- EPA:
-
Eicosapentaenoic acid
- DPA:
-
Docosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- 8-HOME:
-
8-Hydroxy-octadecanoic acid
- 10-HOME:
-
10-Hydroxy-octadecanoic acid
- 11-HOME:
-
11-Hydroxy-octadecanoic acid
- 13-HODE:
-
13-Hydroxy-octadecadienoic acid
- 9-HOTrE:
-
9-Hydroxy-octadecatrienoic acid
- 5-HETE:
-
5-Hydroxy-eicosapentaenoic acid
- 12-HETE:
-
12-Hydroxy-eicosapentaenoic acid
- 15-HETE:
-
15-Hydroxy-eicosapentaenoic acid
- 10-HDPE:
-
10-Hydroxy-docosapentenoic acid
- 10-HDoHE:
-
10-Hydroxy-docosahexaenoic acid
- 9-HpOME:
-
9-Hydroperoxy-octadecanoic acid
- 10-HpOME:
-
10-Hydroperoxy-octadecanoic acid
- 9-HpODE:
-
9-Hydroperoxy-octadecadienoic acid
- 13-HpODE:
-
13-Hydroperoxy-octadecadienoic acid
- 9-HpOTrE:
-
9-Hydroperoxy-octadecatrienoic acid
- 9-KOME:
-
9-Oxo-octadecanoic acid
References
FAO (2020) The state of world fisheries and aquaculture. Food and Agriculture Organization of the United Nations, Rome
Chiba T, Brash AR, Furue M (2016) The precise structures and stereochemistry of trihydroxy-linoleates esterified in human and porcine epidermis. J Biol Chem 291:14540–14554. https://doi.org/10.1074/jbc.M115.711267
Xie D, Jin J, Sun J et al (2017) Comparison of solvents for extraction of krill oil from krill meal: Lipid yield, phospholipids content, fatty acids composition and minor components. Food Chem 233:434–441. https://doi.org/10.1016/j.foodchem.2017.04.138
Maríad G, Encarnación G (2009) Oxidation of corn oil at room temperature: primary and secondary oxidation products and determination of their concentration in the oil liquid matrix from 1H nuclear magnetic resonance data. Food Chem 116:183–192. https://doi.org/10.1016/j.foodchem.2009.02.029
Morales A, Marmesat S, Dobarganes MC et al (2012) Quantitative analysis of hydroperoxy-, keto- and hydroxy-dienes in refined vegetable oils. J Chromatogr A 1229:190–197. https://doi.org/10.1016/j.chroma.2012.01.039
Giua L, Blasi F, Simonetti MS et al (2013) Oxidative modifications of conjugated and unconjugated linoleic acid during heating. Food Chem 140:680–685. https://doi.org/10.1016/j.foodchem.2012.09.067
Nogueira MS, Scolaro B, Milne GL et al (2018) Oxidation products from omega-3 and omega-6 fatty acids during a simulated shelf life of edible oils. LWT-Food Sci Technol 101:113–122. https://doi.org/10.1016/j.lwt.2018.11.044
Cao J, Jiang X, Chen Q et al (2020) Oxidative stabilities of olive and camellia oils: possible mechanism of aldehydes formation in oleic acid triglyceride at high temperature. LWT-Food Sci Technol 118:108858. https://doi.org/10.1016/j.lwt.2019.108858
Hu XF, Li JL, Zhang L et al (2022) Effect of frying on the lipid oxidation and volatile substances in grass carp (Ctenopharyngodon idellus) fillet. J Food Process Preserv 00:e16342. https://doi.org/10.1111/jfpp.16342
Liu X, Wang S, Masui E et al (2020) Model for prediction of the carbonyl value of frying oil from the initial composition. LWT-Food Sci Technol 117:108660. https://doi.org/10.1016/j.lwt.2019.108660
Mehta BM, Darji VB, Aparnathi KD (2015) Comparison of five analytical methods for the determination of peroxide value in oxidized ghee. Food Chem 185:449–453
Li JL, Tu ZC, Sha XM et al (2016) Effect of frying on fatty acid profile, free amino acids and volatile compounds of grass carp (ctenopharyngodon idellus) fillets. J Food Process Preserv 41:e13088. https://doi.org/10.1111/jfpp.13088
Choe E, Min DB (2020) Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci Food Safety 5:169–186. https://doi.org/10.1111/j.1541-4337.2006.00009.x
Parmar P, Kaushik K, Devaraja HC et al (2013) Journal of medicinal plants research the effects of alcoholic extract of Arjuna (Terminalia arjuna Wight & Arn.) bark on stability of clarified butterfat. J Med Plant Res 7:2545–2550. https://doi.org/10.5897/JMPR2013.5114
Farhoosh R, Khodaparast M, Sharif A et al (2012) Olive oil oxidation: rejection points in terms of polar, conjugated diene, and carbonyl values. Food Chem 131:1385–1390. https://doi.org/10.1016/j.foodchem.2011.10.004
Serhan CN, Levy BD (2018) Omega-3 fatty acid-derived mediators that control inflammation and tissue homeostasis emergence of the pro-resolving superfamily of mediators. J Clin Investig 128:2657–2669. https://doi.org/10.1172/JCI97943
Chen J, Jayachandran M, Bai W et al (2022) A critical review on the health benefits of fish consumption and its bioactive constituents. Food Chem 369:130874. https://doi.org/10.1016/j.foodchem.2021.130874
Wang C, Xia W, Xu Y et al (2014) Comparative study on nutritional value and fatty acid profiles of brains and eyes from four freshwater fishes. J Am Oil Chem Soc 91:1471–1476. https://doi.org/10.1007/s11746-014-2449-7
Xu J, Yan B, Teng Y et al (2010) Analysis of nutrient composition and fatty acid profiles of Japanese sea bass lateolabrax japonicus (cuvier) reared in seawater and freshwater. J Food Compos Anal 23:401–405. https://doi.org/10.1016/j.jfca.2010.01.010
Foret MK, Lincoln R, Carmo SD et al (2020) Connecting the “dots”: from free radical lipid autoxidation to cell pathology and disease. Chem Rev 120:12757–12787. https://doi.org/10.1021/acs.chemrev.0c00761
Lehotay SJ, Sapozhnikova Y, Mol HGJ (2015) Current issues involving screening and identification of chemical contaminants in foods by mass spectrometry. TrAC, Trends Anal Chem 69:62–75. https://doi.org/10.1016/j.trac.2015.02.012
Song G, Zhang M, Zhang Y et al (2019) In situ method for real-time discriminating salmon and rainbow trout without sample preparation using iknife and rapid evaporative ionization mass spectrometry-based lipidomics. J Agric Food Chem 67:4679–4688. https://doi.org/10.1021/acs.jafc.9b00751
Rincón-Cervera MÁ, González-Barriga V, Valenzuela R et al (2019) Profile and distribution of fatty acids in edible parts of commonly consumed marine fishes in Chile. Food Chem 274:123–129. https://doi.org/10.1016/j.foodchem.2018.08.113
Wang X, Zhang H, Song Y et al (2019) Comparative lipid profile analysis of four fish species by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J Agric Food Chem 67:9423–9431
Zhang D, Li X, Duan X et al (2021) Lipidomics reveals the changes in lipid profile of flaxseed oil affected by roasting. Food Chem 364:130431
Ito J, Shimizu N, Kobayashi E et al (2017) A novel chiral stationary phase LC-MS/MS method to evaluate oxidation mechanisms of edible oils. Sci Rep 7:10026. https://doi.org/10.1038/s41598-017-10536-2
Oliw EH, Wennman A, Hoffmann I et al (2011) Stereoselective oxidation of regioisomeric octadecenoic acids by fatty acid dioxygenases. J Lipid Res 52:1995–2004. https://doi.org/10.1194/jlr.M018259
Du ZY, Jian Z, Wang C et al (2012) Risk–benefit evaluation of fish from Chinese markets: nutrients and contaminants in 24 fish species from five big cities and related assessment for human health. Sci Total Environ 416:187–199. https://doi.org/10.1016/j.scitotenv.2011.12.020
Yin H, Xu L, Porter NA (2011) Free radical lipid peroxidation: mechanisms and analysis. Chem Rev 111:5944–5972
Zahradka P, Neumann S, Aukema HM et al (2017) Adipocyte lipid storage and adipokine production are modulated by lipoxygenase-derived oxylipins generated from 18-carbon fatty acids. Int J Biochem Cell Biol 88:23–30. https://doi.org/10.1016/j.biocel.2017.04.009
Pauls SD, Rodway LA, Winter T et al (2018) Anti-inflammatory effects of α-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from α-linolenic and linoleic acid. J Nutr Biochem 57:121–129. https://doi.org/10.1016/j.jnutbio.2018.03.020
Isobe Y, Arita M (2014) Identification of novel omega-3 fatty acid-derived bioactive metabolites based on a targeted lipidomics approach. J Clin Biochem Nutr 55:79–84. https://doi.org/10.3164/jcbn.14-18
Zhang Q, Qin W, Lin D et al (2015) The changes in the volatile aldehydes formed during the deep-fat frying process. J Food Sci Technol 52:7683–7696. https://doi.org/10.1007/s13197-015-1923-z
Jiang Y, Zhao L, Yuan M et al (2017) Identification and changes of different volatile compounds in meat of crucian carp under short-term starvation by GC-MS coupled with HS-SPME. J Food Biochem 41:e12375. https://doi.org/10.1111/jfbc.12375
Chang C, Wu G, Zhang H et al (2020) Deep-fried flavor: characteristics, formation mechanisms, and influencing factors. Crit Rev Food Sci Nutr 60:1496–1514. https://doi.org/10.1080/10408398.2019.1575792
Peroni E, Scali V, Balestri F et al (2020) Pathways of 4-hydroxy-2-nonenal detoxification in a human astrocytoma cell line. Antioxidants 9:385. https://doi.org/10.3390/antiox9050385
Fratini G, Lois S, Pazos M et al (2012) Volatile profile of Atlantic shellfish species by HS-SPME-GC/MS. Food Res Int 48:856–865. https://doi.org/10.1016/j.foodres.2012.06.033
Lloyd MA, Drake MA, Gerard PD (2009) Flavor variability and flavor stability of U.S.-produced whole milk powder. J Food Sci 74:S334–S343. https://doi.org/10.1111/j.1750-3841.2009.01299.x
Ba HV, Ryu KS, Lan NTK et al (2013) Influence of particular breed on meat quality parameters, sensory characteristics, and volatile components. Food Sci Biotechnol 22:651–658. https://doi.org/10.1007/s10068-013-0127-4
Acknowledgements
This work was supported by the Chinese National Natural Science Foundations (NO. 32060557), the open fund of State Key Laboratory of Food Science and Technology, Nanchang University (NO. SKLF-KF-202017). Key Research and Development projects in Jiangxi Province (NO. 20203BBFL63062). Key Research and Development projects in Jiujiang City, Jiangxi Province (NO. S2021DYFN076).
Author information
Authors and Affiliations
Contributions
Xiangfei Hu: Conceptualization, Methodology, Formal analysis, Visualization. Bin Peng: Data curation, Writing review and editing. Shuanglong Wang: Supervision, Data curation. Zongcai Tu: Supervision, Funding acquisition, Data curation. Jinlin Li: Supervision, Funding acquisition, Data curation. Hui Wang: Investigation. Yueming Hu: Investigation. Bizhen Zhong: Investigation.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Oxidative stabilities of grass carp oil: possible mechanisms of volatile species formation in hydroperoxylated metabolites at high temperature.”
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiangfei Hu and Bin Peng are co-first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, X., Peng, B., Wang, S. et al. Oxidative stabilities of grass carp oil: possible mechanisms of volatile species formation in hydroperoxylated metabolites at high temperature. Eur Food Res Technol 248, 2079–2095 (2022). https://doi.org/10.1007/s00217-022-04032-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04032-9