Abstract
The stability of tea phenolic compounds is influenced by pH value and digestive processes. However, the complex mixture of constituents in tea may modulate the stability of these compounds during digestion. In this study, tea infusions obtained from green, black, and Oolong tea leaves were exposed to in vitro simulated gastrointestinal digestion, and the stability of ( +)-catechin, caffeine, (−)-epicatechin, epigallocatechin-3-gallate (EGCG), and gallic acid was compared to that of isolated compounds. Changes in antioxidant activity were also evaluated by means of DPPH assay and in a H2O2-induced in vitro oxidative stress model, using Caco-2 cells. The stability of teas antioxidant constituents was different when using teas extract, compared to the reference compound alone, with the total phenolic content being more stable in extracts containing them in higher amount. EGCG degradation correlated well with changes in the DPPH inhibition assay, confirming its pivotal role in the antioxidant activity of tea. Differently, the antioxidant effect in the in vitro cell-based model was much more related to the initial total phenolic content of the extracts, with green tea being more effective than black tea and Oolong tea. Moreover, the antioxidant activity of teas was strongly affected by gastrointestinal digestion. Taken together, these findings suggest a protective role of teas phytocomplex against gastrointestinal digestion of antioxidant constituents. In conclusion, the effect of gastrointestinal digestion on the antioxidant activity of tea should be taken into account, as this may be different from one extract to another and information on the stability of active constituents cannot be extrapolated from data obtained using single compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxidative stress is a major concern of modern medicine, and has been reported to cause harmful effects on cellular structures in human tissues, such as proteins, lipids, and nucleic acids [1,2,3,4,5,6]. These phenomena can lead to the onset of several diseases, including cancer, stroke, and neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases and multiple sclerosis [7,8,9,10].
Camellia sinensis (L.) Kuntze leaves have been used for centuries to produce tea. Once harvested, differences in the manufacturing process of C. sinensis leaves lead to different kind of tea, with green, black, and Oolong tea being the most used worldwide [11]. Green tea is produced by using freshly harvested leaves, which are immediately steamed or pan-fried to prevent polyphenols oxidation, thus inhibiting fermentation. Differently, black and Oolong tea are typically produced using fully and semi-fermented withered rolled leaves, respectively [12, 13].
Tea is one of the most popular beverage rich in antioxidants and its bioactivity, together with that of its main constituents, has been observed in several in vitro and in vivo models [14,15,16].
The chemical composition of C. sinensis leaves has been deeply investigated, and is known to be influenced by leaf processing steps [17]. Among the bioactive components of tea, flavan-3-ols (catechins), a group of phenolic compounds, are the most abundant [18,19,20]. They can be found as flavonol monomers (i.e. catechin, epicatechin) and as flavonol gallates (i.e., epigallocatechin gallate) and their content varies during the fermentation process, due to oxidation or condensation reactions [21, 22]. Epigallocatechin-3-gallate (EGCG) is the most abundant flavan-3-ol in most kind of teas. In addition, the presence of other polyphenols, such as gallic acid, chlorogenic acid, ellagic acid, and flavonols has been reported, with gallic acid being the most common. Theaflavins are present in smaller amount in fermented teas [23]. Caffeine is another characteristic component of tea and is present in considerable amount, almost regardless of the kind of tea [24,25,26].
The effect of gastrointestinal digestion on the content of different tea polyphenols has been reported recently [27]. Moreover, the bioaccessibility and bioavailability of tea catechins are modulated by the co-administration of food and common beverage additives, such as citric acid, ascorbic acid, milk, and citrus juice [28, 29]. In addition, tea infusions and EGCG were found to modulate the stability during digestion of other food ingredients, such as soybean emulsion and starch [30,31,32,33]. In vitro simulated digestion was reported to influence the antimicrobial activity of green tea [34]. The changes in the secretion of cholecystokinin and glucagon-like peptide induced by green tea in vitro was also found to be modulated by simulated digestion [35]. Nevertheless, information regarding the effect of digestion on the bioactivity of tea, such as the antioxidant activity, is scarce. In 2015, Jilani and co-authors investigated the effect of biosorption of green and black tea polyphenols into Saccharomyces cerevisiae on their antioxidant activity before and after digestion, reporting an important loss of total phenolic content (TPC) and flavan-3-ols content [36]. The antioxidant activity, measured using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and expressed as Trolox equivalent antioxidant capacity, was reduced for green tea, and increased for black tea after in vitro simulated gastrointestinal digestion. The same research group used a similar approach to evaluate the effect of biosorption into S. cerevisiae on the antiproliferative activity of digested green and black tea in Caco-2 cells. Among the observed effects, a reduction of the production of reactive oxygen species was highlighted, even if no comparison between pre- and post-digested teas was performed [37]. Finally, in 2021, Chen and co-workers showed a decrease in the antioxidant capacity and TPC of nine commercially available tea juices after in vitro simulated digestion, using cell-free methods [38].
The complexity of tea composition may influence the stability of its constituents upon gastrointestinal digestion, thus affecting the antioxidant activity, and this effect can differ based on the kind of tea used.
To verify this hypothesis, we performed in vitro simulated gastrointestinal digestion on different C. sinensis leaves infusions and we evaluated the influence of digestion on the antioxidant activity of tea using a validated cell-free method and an in vitro model of intestinal epithelial cells.
Materials and methods
Sample preparation and chemical analysis
Green, black, and Oolong tea leaves were purchased from Erbamea (San Giustino, Perugia, Italy). Leaves (3 g) were extracted with 30 mL of ultrapure double-distilled water (ddH2O) (Drug Extract Ratio, DER, 1:10) at 90 °C for 10 min. The extraction time was prolonged to 10 min to maximize the TPC and the extraction of flavan-3-ols [39]. Green tea extract (GTE), black tea extract (BTE), and Oolong tea extract (OTE) were then filtered, and their chemical profile was analyzed by means of HPLC–DAD, using a Shimadzu Prominence LC 2030 3D instrument. A Bondpak® C18 column, 10 µm, 125 Å, 3.9 mm × 300 mm (Waters Corporation, Milford, MA), was used as stationary phase. The mobile phase was composed of water with 0.1% V/V formic acid (A) and acetonitrile with 0.1% V/V formic acid (B), using the following gradient phases: from 10 to 25% in 15 min, from 25 to 35% in 3 min, and from 35 to 50% in 7 min. The flux was set to 0.8 mL/min and the injected volume was 10 µL. Absorbance was recorded at 280 nm and calibration curves using gallic acid, ( +)-catechin, caffeine, (−)-epicatechin, and EGCG as reference standards (Sigma-Aldrich, Milan, Italy), ranging from 0.008 to 0.5 mg/mL (R2 > 0.99), were used to quantify the amount of teas constituents.
The TPC was evaluated with the Folin-Ciocalteu method, as previously described [40].
In vitro simulated digestion
In vitro simulated digestion was carried out as previously described [41], with some modifications. Briefly, 1 mL of GTE, BTE, or OTE was diluted in 19 mL of simulated gastric juice, containing pepsin from porcine gastric mucosa (300 UI/mL, Sigma-Aldrich) and NaCl (10 mg/mL). The pH of the solution was adjusted to 1.7 using HCl. Gallic acid, ( +)-catechin, caffeine, (−)-epicatechin, and EGCG were independently diluted in the same solution to the final concentration of 1 mg/mL. Samples were incubated for 2 h at 37 °C with shaking. Then, pancreatin from porcine pancreas (activity equivalent to 4 × U.S.P., 10 mg/mL, Sigma-Aldrich) and bile salts mixture (20 mg/mL, Sigma-Aldrich) were added, and the pH was increased to 7.0 by adding NaHCO3 (15 mg/mL, Sodalco S.p.A., Corsico, Italy) to simulate the intestinal environment. Intestinal digestion was carried out for 2 h at 37 °C with shaking. Samples were then filtered and immediately used for further analysis.
The relative gastrointestinal stability was calculated as the % recovery of the sample after digestion, compared to the initial amount of sample used.
Antioxidant activity measured using a DPPH test
The radical scavenging activity of the pre- and post-digestion samples was measured by means of the DPPH assay, as previously reported [42]. Briefly, 10 µL of different concentrations (0.16–10 mg/mL) of the samples were added to 190 µL of freshly prepared methanolic DPPH solution (0.1 mM) and incubated for 30 min at rt in the dark with shaking. Then, absorbance was recorded at 517 nm using a Victor® Nivo™ plate reader (PerkinElmer, Waltham, MA). ddH2O was used as the blank control. The antiradical activity of the samples was calculated according to the following formula:
Data were plotted using Microsoft Excel and the IC50 (µg/mL) was determined for each sample.
Cell culture
Intestinal epithelial cells (Caco-2), a kind gift from Prof. Monica Montopoli (University of Padua, Italy) were cultured in 25 cm2 flasks (Sarstedt, Milan, Italy) in high glucose Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich), supplemented with 10% heat-inactivated fetal bovine serum (FBS, Sigma-Aldrich), 1% glutamine (Sigma-Aldrich) and 1% penicillin/streptomycin solution (Sigma-Aldrich). EDTA-trypsin (Sigma-Aldrich) solution was used for detaching cells from flasks, and cell counting was performed using a hemocytometer by Trypan Blue staining. Cell passage number was kept between 30 and 40.
Determination of antioxidant activity in H2O2-stimulated Caco-2 cells
Caco-2 cells (1 × 104) were seeded into 96-well plates and allowed to grow to confluence (70–80%). Cells were then pre-treated overnight with pre- and post-digestion GTE, BTE, and OTE (diluted 1:1000, according to previously performed cell viability assays) and oxidative stress was induced by administering 5 mM hydrogen peroxide (H2O2) for 6 h. Medium was then removed, and cells were washed three times with phosphate buffered saline (PBS). Then, 100 µL of Cell Counting Kit (CCK-8, Sigma-Aldrich) solution (1:100 in RPMI without phenol red) were added to each well and incubated for 1 h at 37 °C. Absorbance was recorded at 450 nm using a Victor® Nivo™ plate reader. Treatments were performed in six replicates, in three independent experiments, and cell viability was calculated by normalizing the absorbance of the test wells to the untreated control.
Measurement of trans-epithelial electric resistance
The efficiency of the intestinal barrier functions was evaluated by measuring trans-epithelial electric resistance (TEER) using a voltmeter [43]. Caco-2 cells (8 × 105) were placed in transparent polyester membrane cell culture inserts with 0.4 μm pore size (Sarstedt), and cultured in 24-well plates, as previously described [44]. Culture medium was replaced every other day. The integrity of the cell monolayers was monitored by measuring the TEER of the monolayer from day 14th to day 21st after seeding. When a stable value was reached, a 12 h pre-treatment was done by adding pre- and post-digested samples (diluted 1:1000) in the apical chamber in appropriate wells and TEER was measured after 0 and 12 h. Then, 0.5 mM H2O2 was added to the basal chamber and TEER was measured after 0, 4, 8, and 24 h. TEER measurements were performed in HBSS with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 10 mM d-glucose (pH = 7.4), after an equilibration period at rt, using a Millicell® ERS meter, (Millipore Corporation, Bedford, MA) connected to a pair of chopstick electrodes. Only cells with TEER value between 360 and 500 Ω cm2 were used for the experiments [45, 46]. Treatments were performed in duplicate in three independent experiments and TEER was expressed as percentage of resistance, normalized to initial value.
DPPH-HPLC-DAD analysis
The DPPH-HPLC-DAD analysis was performed as described elsewhere [47]. Briefly, 100 µL of each sample were added to 300 µL of 10 mM DPPH solution and incubated for 30 min at rt in the dark, with shaking. Samples were then filtered and analyzed by means of HPLC-DAD, using the same chromatographic conditions described in the “Sample preparation and chemical analysis” paragraph.
Statistical analysis
The statistical differences between the biological results were determined by the analysis of the variance (ANOVA). Pearson linear correlation test was used to analyze the correlation between TPC and DPPH. Values are expressed as mean values ± standard deviation, and the differences between the means were considered statistically significant at p < 0.05. Graphs and calculations were performed using GraphPad Prism.
Results and discussion
Chemical analysis
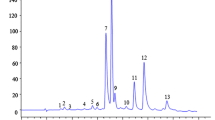
Gallic acid (retention time (RT) = 4.5 min), ( +)-catechin (RT = 7.2 min), caffeine (RT = 8.3 min), and EGCG (RT = 10.4 min) were identified in each sample by comparison of their RT and UV spectra with those of reference standards. (−)-Epicatechin (RT = 9.0 min) was quantifiable only in GTE.
The chemical composition of the samples is reported in Table 1. BTE and OTE contain comparable amount of gallic acid and ( +)-catechin, whereas, ECGC was significantly higher in BTE. The amount of gallic acid and ( +)-catechin was lower in GTE, compared to the other two samples. This is partially consistent with the results of Sun and co-authors, who observed lower amount of gallic acid and higher levels of ( +)-catechin in green tea, in comparison to black and Oolong teas [48]. On the contrary, a significantly higher content of EGCG was found in GTE, compared to the other samples. (−)-epicatechin was quantifiable only in GTE, whereas, caffeine content was comparable in all the samples. The higher amount of EGCG in GTE, compared to BTE, is consistent with the data reported by Tenore and co-authors, who, on the contrary, observed higher amount of ( +)-catechin in green tea, compared to black tea [49]. However, in a more recent work, the same research group reported lower amount of ( +)-catechin in green tea, compared to black tea, which is coherent with our data [27].
Several other flavanols could be found in tea extracts, and their amount and number may vary depending on the extraction method used. In this work, we focused on the main antioxidant constituent (i.e., EGCG), which is present in all the studied extracts, and its main parent compounds and metabolites. As caffeine is also present in high amount in all the samples, and it belongs to a different class of polyphenols, we decided to include it in this study.
Stability of tea constituents under in vitro simulated digestion
Stability of tea constituents resulted to be significantly influenced by in vitro simulated digestion (Table 2). Indeed, the recovery of flavan-3-ols, tested as single reference standards, after the digestion process was very low, with EGCG being the most affected among the three flavan-3-ols tested. These data are consistent with that obtained by Krook and Hagerman, who observed that, despite being stable at acidic pH similar to that of the stomach, 90% of EGCG was decomposed after intestinal digestion [50]. Similarly, Yoshino and co-authors found that 80% of EGCG was already decomposed after 5 min in authentic intestinal juice [51]. In our work, we employed longer incubation time than that used in these two studies (i.e., 2 h of intestinal digestion phase, compared to 1 h and 5 min, respectively), which explains a much more evident reduction of EGCG. Interestingly, (−)-epicatechin was reported to be more stable than EGCG at pH = 7 [52], with a recovery of 90% after 17 h. We found that, although being higher than that of EGCG, the recovery of ( +)-catechin and (−)-epicatechin after the whole gastrointestinal digestion process were 27.29% and 13.13%, respectively. Gallic acid recovery was also low and comparable to that of (−)-epicatechin. Caffeine was completely recovered, suggesting a very high stability to gastrointestinal digestion.
The TPC recovery was also significantly reduced by the gastrointestinal digestion, with GTE showing the highest stability (Fig. 1a). Indeed, the relative gastrointestinal stability of GTE, BTE, and OTE was 61.29% ± 6.45%, 55.77% ± 7.69% and 42.11% ± 2.63%, respectively, with OTE recovery being significantly lower in comparison to GTE. Jilani and co-authors reported that the TPC of green and black teas after digestion was 85% and 96% of the initial value, respectively [36]. While the higher reduction of TPC observed by us could be related to differences in the applied model of simulated digestion, the increased stability of black tea TPC, compared to green tea is not consistent with our results. This could be due to differences in the chemical composition of BTE, compared to the black tea used by these authors, which is possibly related to the use of black teas from different sources. Indeed, Annunziata and co-authors obtained a higher duodenal bioaccessibility of green tea TPC (24%), compared to black tea TPC (13%), using HPLC-DAD for quantification [27].
a Relative gastrointestinal stability of GTE, BTE, and OTE TPC. ***p < 0.001 vs. reference standard; ° p < 0.05 vs. GTE; one-way ANOVA followed by Dunnett’s post-hoc. b Relative gastrointestinal stability of GTE, BTE, and OTE main constituents. *p < 0.05 vs. reference standard; **p < 0.01 vs. reference standard; ***p < 0.001 vs. reference standard; two-way ANOVA followed by Sídàk’s post-hoc
Interestingly, teas phytocomplex protected flavan-3-ols and gallic acid from the degradation (Fig. 1b). Consistently with the study of Tenore and co-workers, the stability of ( +)-catechin, (−)-epicatechin, and EGCG was higher in GTE, compared to BTE [49]. The higher percentage recovery of flavan-3-ols in GTE and BTE obtained in our work could be due to differences in the simulated digestion model used and in the chemical composition of the samples. Indeed, ( +)-catechin recovery after simulated digestion was approximately 100% in BTE and OTE and approximately 160% in GTE. (−)-Epicatechin was not found in BTE and OTE, thus, its stability to digestion was not quantified in these extracts. However, (−)-epicatechin completely disappeared after simulated digestion of GTE. A possible explanation of the increased recovery of ( +)-catechin in digested GTE may be related to the partial degradation of flavan-3-ols oligomers and conversion of (−)-epicatechin to catechin, which may occur in aqueous solution as a consequence of temperature and pH changes [53, 54]. The gastrointestinal stability of EGCG was dependent on the tea sample, suggesting that different phytocomplexes may specifically influence its recovery after digestion. Indeed, EGCG recovery was approximately 65%, 15%, and 42% in GTE, BTE, and OTE, respectively, with the recovery from BTE being significantly lower compared to GTE and OTE. Interestingly, the relative recovery of EGCG contained in the test teas was always significantly higher compared to the reference standard alone. Similarly, gallic acid stability to digestion was different for each sample, with BTE resulting in the highest recovery and OTE in the lowest. The increased recovery of gallic acid in teas, compared to the reference standard alone, may result from the hydrolysis of the galloyl moiety from galloyl catechins such as catechin-gallate, gallocatechin-gallate, epicatechin-3-gallate, and EGCG [48, 50]. Consistently with this explanation, in fact, the recovery of gallic acid was higher in BTE, and was accompanied by a lower recovery of EGCG. Caffeine recovery was around 100% in each tea sample, similarly to its reference standard. Interestingly, caffeine was reported to modulate the oral bioavailability of EGCG in humans: beverages containing higher amount of EGCG than caffeine showed higher EGCG bioavailability compared to those containing higher amount of caffeine than EGCG [55]. These observations are consistent with our results in which GTE, which contained more EGCG than caffeine, showed a higher gastrointestinal stability of EGCG in comparison to BTE, which contained similar amount of EGCG and caffeine, and to OTE, which contained more caffeine than EGCG.
One limitation of this study is represented by the in vitro simulated digestion model used. Indeed, differences in the results obtained using variations of this method are common across different laboratories, highlighting the need for a unified method. An attempt to standardize the in vitro static digestion method is being carried out by the INFOGEST COST action, which is continuously updating the guidelines for setting up a standardized static digestion model suitable for foods analysis [56, 57]. Although this method was not used in the present work, our results are consistent with those obtained by other research groups, suggesting the reliability of our approach.
The antioxidant activity of teas is reduced by digestion
The DPPH assay was used as a fast and simple method to evaluate the effect of gastrointestinal digestion on the antioxidant activity of teas. The radical scavenging potential of pre-digestion samples (expressed as IC50 values in Table 3) was less than 600 µg/mL, with GTE being the most active. Simulated digestion significantly increased the IC50 values of GTE, BTE, and OTE by 2.3-, 9.6-, and 5.5-fold, respectively, thus suggesting a negative effect of digestion on antioxidant potential. When comparing the antioxidant activity of green, black, and white teas using the DPPH assay, Annunziata and co-authors found that in vitro simulated digestion had a stronger negative effect on black tea radical scavenging activity, compared to green tea, which is consistent with our results [27].
The Pearson’s linear correlation test between the TPC and DPPH of the samples after gastrointestinal digestion showed a good, though not statistically significant, correlation (r = 0.99, p = 0.062). Similarly, a strong correlation (r = 0.99, p = 0.071) was found between the DPPH/TPC ratio of the undigested and digested samples. The high p values obtained despite the good r values are likely dependent on the sample size used (N = 3).
By combining pre-column DPPH assay with HPLC–DAD analysis, it is possible to observe which of the identified teas constituent is predominantly involved in the radical scavenging activity of the samples. Indeed, upon reaction with DPPH, the peak area of the anti-radical compounds should decrease or disappear in the HPLC-DAD chromatogram [47, 58, 59]. Figure 2 shows the complete disappearance of peaks area corresponding to gallic acid, ( +)-catechin, (−)-epicatechin, and EGCG, thus suggesting their pivotal role for the antioxidant activity of teas. Differently, caffeine peak was smaller but still present in the post-DPPH, compared to the pre-DPPH chromatogram (approximately −25% of the initial peak area).
In GTE, only EGCG recovery was significantly lower after simulated digestion, compared to the other tested constituents. This result, combined with the increased level of gallic acid and ( +)-catechin, may account for the relatively small increase in DPPH IC50. EGCG stability in BTE was the most affected by simulated digestion among the three different samples. This is consistent with the increase in DPPH IC50, which is the highest, compared to the other teas. OTE can be distinguished from the other teas, because of the strong reduction of gallic acid levels after simulated digestion. EGCG, however, was more stable in OTE, compared to BTE, thus explaining why the DPPH IC50 was less affected, in terms of fold changes, compared to BTE, but more affected compared to GTE.
Caco-2 cells have been used as a stable and reliable model of intestinal epithelium [60, 61]. 5 mM H2O2 induced a significant oxidative damage to Caco-2 cells, reducing cell viability by approximately 20% compared to the untreated control after 6 h. The pre-treatment with GTE, BTE, and OTE significantly protected Caco-2 cells from the cytotoxic effect of H2O2, with GTE being the most effective extract. The antioxidant activity, in terms of Caco-2 protection, of GTE after in vitro digestion was slightly reduced compared to the pre-digestion sample, although the difference was not statistically significant. On the contrary, BTE and OTE activity was significantly reduced after simulated digestion, with OTE completely losing its protective effect (Fig. 3a). Consistently, the prolonged stimulation with lower concentration of H2O2 (0.5 mM) caused a time-dependent alteration of the intestinal barrier integrity, with TEER values being reduced by approximately 45% after 24 h. GTE, BTE, and OTE significantly reduced the oxidative damage provoked by H2O2, with similar effectiveness compared to the cell viability test. The protection from the H2O2-impaired intestinal barrier integrity of each sample was reduced after simulated digestion, even if statistical significance was obtained only for OTE (Fig. 3b).
Effect of simulated digestion on the antioxidant activity of GTE, BTE, and OTE in Caco-2 cells. a: Protection from H2O2-impaired cell viability. Data are expressed as percentage of cell viability compared to untreated cells. The effect of H2O2 is represented by the dashed line. b: Protection of the intestinal barrier function observed by transepithelial electrical resistance measurement. ###p < 0.001 vs. untreated control; **p < 0.01 vs. stimulus; ***p < 0.001 vs. stimulus; °p < 0.05 vs. pre-digestion sample; °°p < 0.01 vs. pre-digestion sample; two-way ANOVA followed by Dunnett’s post-hoc
Contrarily to the DPPH assay, the results obtained in the cell-based experiments suggest that the reduction of Caco-2 protection from the oxidative damage is not directly dependent on the degree of stability of teas main constituents alone, but rather depends on the initial amount of antioxidant constituents (i.e., TPC) in the samples. Indeed, in the cell viability assay, the post-digestion antioxidant activity was reduced by 7%, 8%, and 10% in GTE, BTE, and OTE, respectively, compared to the pre-digestion samples. Moreover, in the TEER experiment, the post-digestion protection of the intestinal barrier integrity was reduced by 14%, 7%, and 23% in GTE, BTE, and OTE, respectively, compared to the pre-digestion samples. To the best of our knowledge, this is the first study evaluating the effects of in vitro simulated digestion on the antioxidant activity of different teas, using the H2O2-impaired Caco-2 cell viability model. Using the same model, Song and Gao reported the protective effect of Fuzhuan brick-tea, without performing gastrointestinal digestion, and found that this protective effect was related to a reduction of lipid peroxidation and to the increase of glutathione and antioxidant enzymes [15]. However, information on the chemical composition of the used tea was not reported, making their results difficult to compare with ours.
Conclusion
Phenolic compounds are a major class of phytochemicals with known antioxidant properties. In this study, the effect of in vitro simulated gastrointestinal digestion on the antioxidant effect of three different C. sinensis extracts was evaluated. The stability of teas main constituents was influenced by the initial extract composition, with TPC being more stable when present in higher amount. EGCG degradation correlated well with changes in the DPPH inhibition assay, thus confirming its pivotal role in the antioxidant activity of tea. Differently, the antioxidant effect in the in vitro cell-based model was much more related to the initial TPC content of the extracts, with green tea being more effective than black tea and Oolong tea. Overall, gastrointestinal digestion strongly affected the biological effectiveness of teas. Interestingly, the stability of teas antioxidant constituents was different when using teas extract, compared to the reference compound alone, thus suggesting a protective role of the phytocomplex, which could lead to increased biological effectiveness. In conclusion, the effect of gastrointestinal digestion should be considered when evaluating the biological properties of herbal extracts, as this may be different from one extract to another and information on the stability of active constituents during the digestion process cannot be extrapolated from data obtained using single compounds.
Data availability
Not applicable.
Code availability
Not applicable.
References
Bhatti JS, Bhatti GK, Reddy PH (2017) Mitochondrial dysfunction and oxidative stress in metabolic disorders—a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis 1863:1066–1077. https://doi.org/10.1016/j.bbadis.2016.11.010
Liguori I, Russo G, Curcio F et al (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757–772. https://doi.org/10.2147/CIA.S158513
Sharifi-Rad M, Anil Kumar NV, Zucca P et al (2020) Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front Physiol 11:694. https://doi.org/10.3389/fphys.2020.00694
Forman HJ, Zhang H (2021) Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 20:689–709. https://doi.org/10.1038/s41573-021-00233-1
Pizzino G, Irrera N, Cucinotta M et al (2017) Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017:8416763. https://doi.org/10.1155/2017/8416763
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247. https://doi.org/10.1038/35041687
Klaunig JE (2018) Oxidative stress and cancer. Curr Pharm Des 24:4771–4778. https://doi.org/10.2174/1381612825666190215121712
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57:1105–1121. https://doi.org/10.3233/JAD-161088
Puspita L, Chung SY, Shim J-W (2017) Oxidative stress and cellular pathologies in Parkinson’s disease. Mol Brain 10:53. https://doi.org/10.1186/s13041-017-0340-9
Tobore TO (2021) Oxidative/nitroxidative stress and multiple sclerosis. J Mol Neurosci 71:506–514. https://doi.org/10.1007/s12031-020-01672-y
Zhang L, Ho C-T, Zhou J et al (2019) Chemistry and biological activities of processed Camellia sinensis teas: a comprehensive review. Compr Rev Food Sci Food Saf 18:1474–1495. https://doi.org/10.1111/1541-4337.12479
Hayat K, Iqbal H, Malik U et al (2015) Tea and its consumption: benefits and risks. Crit Rev Food Sci Nutr 55:939–954. https://doi.org/10.1080/10408398.2012.678949
Khan N, Mukhtar H (2018) Tea polyphenols in promotion of human health. Nutrients. https://doi.org/10.3390/nu11010039
Prasanth MI, Sivamaruthi BS, Chaiyasut C, Tencomnao T (2019) A Review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients 11:474. https://doi.org/10.3390/nu11020474
Song J-L, Gao Y (2014) Effects of methanolic extract form Fuzhuan brick-tea on hydrogen peroxide-induced oxidative stress in human intestinal epithelial adenocarcinoma Caco-2 cells. Mol Med Rep 9:1061–1067. https://doi.org/10.3892/mmr.2014.1884
Chen T, Yang Y, Zhu S et al (2020) Inhibition of Aβ aggregates in Alzheimer’s disease by epigallocatechin and epicatechin-3-gallate from green tea. Bioorg Chem 105:104382. https://doi.org/10.1016/j.bioorg.2020.104382
Lee M-K, Kim H-W, Lee S-H et al (2019) Characterization of catechins, theaflavins, and flavonols by leaf processing step in green and black teas (Camellia sinensis) using UPLC-DAD-QToF/MS. Eur Food Res Technol 245:997–1010. https://doi.org/10.1007/s00217-018-3201-6
de Mejia EG, Ramirez-Mares MV, Puangpraphant S (2009) Bioactive components of tea: cancer, inflammation and behavior. Brain Behav Immun 23:721–731. https://doi.org/10.1016/j.bbi.2009.02.013
Musial C, Kuban-Jankowska A, Gorska-Ponikowska M (2020) Beneficial properties of green tea catechins. Int J Mol Sci. https://doi.org/10.3390/ijms21051744
Reygaert WC (2018) Green tea catechins: their use in treating and preventing infectious diseases. Biomed Res Int 2018:9105261. https://doi.org/10.1155/2018/9105261
Chaturvedula VSP, Prakash I (2011) The aroma, taste, color and bioactive constituents of tea. J Med Plants Res 5:2110–2124. https://doi.org/10.5897/JMPR.9001187
Reygaert WC (2017) An update on the health benefits of green tea. Beverages 3:6. https://doi.org/10.3390/beverages3010006
Tang G-Y, Meng X, Gan R-Y et al (2019) Health functions and related molecular mechanisms of tea components: an update review. Int J Mol Sci 20:6196. https://doi.org/10.3390/ijms20246196
Gramza-Michalowska A (2014) Caffeine in tea Camellia sinensis–content, absorption, benefits and risks of consumption. J Nutr Health Aging 18:143–149. https://doi.org/10.1007/s12603-013-0404-1
Bobková A, Demianová A, Belej Ľ et al (2021) Detection of changes in total antioxidant capacity, the content of polyphenols, caffeine, and heavy metals of teas in relation to their origin and fermentation. Foods (Basel, Switzerland). https://doi.org/10.3390/foods10081821
Cabrera M, Taher F, Llantada A et al (2021) Effect of water hardness on catechin and caffeine content in green tea infusions. Molecules. https://doi.org/10.3390/molecules26123485
Annunziata G, Maisto M, Schisano C et al (2018) Colon bioaccessibility and antioxidant activity of white, green and black tea polyphenols extract after in vitro simulated gastrointestinal digestion. Nutrients 10:1711. https://doi.org/10.3390/nu10111711
Green RJ, Murphy AS, Schulz B et al (2007) Common tea formulations modulate in vitro digestive recovery of green tea catechins. Mol Nutr Food Res 51:1152–1162. https://doi.org/10.1002/mnfr.200700086
Choi E-H, Lee D-Y, Kim S et al (2017) Influence of flavonol-rich excipient food (onion peel and Dendropanax morbifera) on the bioavailability of green tea epicatechins in vitro and in vivo. Food Funct 8:3664–3674. https://doi.org/10.1039/c7fo01173c
Fu T, Niu L, Li Y et al (2020) Effects of tea products on in vitro starch digestibility and eating quality of cooked rice using domestic cooking method. Food Funct. https://doi.org/10.1039/d0fo02499f
Li X, Li S, Chen M et al (2018) (-)-Epigallocatechin-3-gallate (EGCG) inhibits starch digestion and improves glucose homeostasis through direct or indirect activation of PXR/CAR-mediated phase II metabolism in diabetic mice. Food Funct 9:4651–4663. https://doi.org/10.1039/c8fo01293h
Ding J, Xu Z, Qi B et al (2018) Physicochemical and oxidative stability of a soybean oleosome-based emulsion and its in vitro digestive fate as affected by (-)-epigallocatechin-3-gallate. Food Funct 9:6146–6154. https://doi.org/10.1039/c8fo01215f
Mika M, Wikiera A, Żyła K (2007) Effects of non-fermented tea extracts on in vitro digestive hydrolysis of lipids and on cholesterol precipitation. Eur Food Res Technol 226:731. https://doi.org/10.1007/s00217-007-0584-1
Marchese A, Coppo E, Sobolev AP et al (2014) Influence of in vitro simulated gastroduodenal digestion on the antibacterial activity, metabolic profiling and polyphenols content of green tea (Camellia sinensis). Food Res Int 63:182–191. https://doi.org/10.1016/j.foodres.2014.01.036
Planes-Muñoz D, López-Nicolás R, González-Bermúdez CA et al (2018) In vitro effect of green tea and turmeric extracts on GLP-1 and CCK secretion: the effect of gastrointestinal digestion. Food Funct 9:5245–5250. https://doi.org/10.1039/c8fo01334a
Jilani H, Cilla A, Barberá R, Hamdi M (2015) Biosorption of green and black tea polyphenols into Saccharomyces cerevisiae improves their bioaccessibility. J Funct Foods 17:11–21. https://doi.org/10.1016/j.jff.2015.05.006
Jilani H, Cilla A, Barberá R, Hamdi M (2020) Antiproliferative activity of green, black tea and olive leaves polyphenols subjected to biosorption and in vitro gastrointestinal digestion in Caco-2 cells. Food Res Int 136:109317. https://doi.org/10.1016/j.foodres.2020.109317
Chen G-L, Hu K, Zhong N-J et al (2013) Antioxidant capacities and total polyphenol content of nine commercially available tea juices measured by an in vitro digestion model. Eur Food Res Technol 236:303–310. https://doi.org/10.1007/s00217-012-1897-2
Kyle JAM, Morrice PC, McNeill G, Duthie GG (2007) Effects of infusion time and addition of milk on content and absorption of polyphenols from black tea. J Agric Food Chem 55:4889–4894. https://doi.org/10.1021/jf070351y
Governa P, Biagi M (2020) Copaifera langsdorffii Desf.: in vitro investigation on anti-Helicobacter pylori and anti-inflammatory activities of oleoresin and fruit methanolic extract. Plant Biosyst An Int J Deal with all Asp Plant Biol 154:117–124. https://doi.org/10.1080/11263504.2019.1578284
McDougall GJ, Fyffe S, Dobson P, Stewart D (2005) Anthocyanins from red wine–their stability under simulated gastrointestinal digestion. Phytochemistry 66:2540–2548. https://doi.org/10.1016/j.phytochem.2005.09.003
Chiocchio I, Poli F, Governa P et al (2019) Wound healing and in vitro antiradical activity of five Sedum species grown within two sites of community importance in Emilia-Romagna (Italy). Plant Biosyst - An Int J Deal with all Asp Plant Biol 153:610–615. https://doi.org/10.1080/11263504.2018.1549611
Catanzaro D, Rancan S, Orso G et al (2015) Boswellia serrata preserves intestinal epithelial barrier from oxidative and inflammatory damage. PLoS ONE 10:e0125375. https://doi.org/10.1371/journal.pone.0125375
Governa P, Marchi M, Cocetta V et al (2018) Effects of Boswellia serrata Roxb. and Curcuma longa L. in an in vitro intestinal inflammation model using immune cells and Caco-2. Pharmaceuticals 11:126. https://doi.org/10.3390/ph11040126
Hubatsch I, Ragnarsson EGE, Artursson P (2007) Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat Protoc 2:2111–2119. https://doi.org/10.1038/nprot.2007.303
Natoli M, Leoni BD, D’Agnano I et al (2012) Good Caco-2 cell culture practices. Toxicol In Vitro 26:1243–1246. https://doi.org/10.1016/j.tiv.2012.03.009
Meda NR, Fraisse D, Gnoula C et al (2017) Characterization of antioxidants from Detarium microcarpum Guill. et Perr. leaves using HPLC-DAD coupled with pre-column DPPH assay. Eur Food Res Technol 243:1659–1666. https://doi.org/10.1007/s00217-017-2873-7
Sun H, Chen Y, Cheng M et al (2018) The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J Food Sci Technol 55:399–407. https://doi.org/10.1007/s13197-017-2951-7
Tenore GC, Campiglia P, Giannetti D, Novellino E (2015) Simulated gastrointestinal digestion, intestinal permeation and plasma protein interaction of white, green, and black tea polyphenols. Food Chem 169:320–326. https://doi.org/10.1016/j.foodchem.2014.08.006
Krook MA, Hagerman AE (2012) Stability of polyphenols epigallocatechin gallate and pentagalloyl glucose in a simulated digestive system. Food Res Int 49:112–116. https://doi.org/10.1016/j.foodres.2012.08.004
Yoshino K, Suzuki M, Sasaki K et al (1999) Formation of antioxidants from (-)-epigallocatechin gallate in mild alkaline fluids, such as authentic intestinal juice and mouse plasma. J Nutr Biochem 10:223–229. https://doi.org/10.1016/s0955-2863(98)00103-x
Henning SM, Choo JJ, Heber D (2008) Nongallated compared with gallated flavan-3-ols in green and black tea are more bioavailable. J Nutr 138:1529S-1534S. https://doi.org/10.1093/jn/138.8.1529S
Komatsu Y, Suematsu S, Hisanobu Y et al (1993) Effects of pH and temperature on reaction kinetics of catechins in green tea infusion. Biosci Biotechnol Biochem 57:907–910. https://doi.org/10.1271/bbb.57.907
Muzolf-Panek M, Gliszczyńska-Świgło A, Szymusiak H, Tyrakowska B (2012) The influence of stereochemistry on the antioxidant properties of catechin epimers. Eur Food Res Technol 235:1001–1009. https://doi.org/10.1007/s00217-012-1826-4
Nakagawa K, Nakayama K, Nakamura M et al (2009) Effects of co-administration of tea epigallocatechin-3-gallate (EGCG) and caffeine on absorption and metabolism of EGCG in humans. Biosci Biotechnol Biochem 73:2014–2017. https://doi.org/10.1271/bbb.90195
Minekus M, Alminger M, Alvito P et al (2014) A standardised static in vitro digestion method suitable for food—an international consensus. Food Funct 5:1113–1124. https://doi.org/10.1039/C3FO60702J
Brodkorb A, Egger L, Alminger M et al (2019) INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Protoc 14:991–1014. https://doi.org/10.1038/s41596-018-0119-1
Qiu J, Chen L, Zhu Q et al (2012) Screening natural antioxidants in peanut shell using DPPH-HPLC-DAD-TOF/MS methods. Food Chem 135:2366–2371. https://doi.org/10.1016/j.foodchem.2012.07.042
Wang G, Huang X, Pei D et al (2016) DPPH-HPLC-DAD analysis combined HSCCC for screening and identification of radical scavengers in Cynomorium songaricum Rupr. New J Chem 40:3885–3891. https://doi.org/10.1039/C5NJ03233D
Cocetta V, Catanzaro D, Borgonetti V et al (2019) A fixed combination of probiotics and herbal extracts attenuates intestinal barrier dysfunction from inflammatory stress in an in vitro model using caco-2 cells. Recent Patents Food, Nutr Agric. https://doi.org/10.2174/2212798410666180808121328
Dallacqua S, Catanzaro D, Cocetta V et al (2016) Protective effects of psi taraxasterol 3-O-myristate and arnidiol 3-O-myristate isolated from Calendula officinalis on epithelial intestinal barrier. Fitoterapia 109:230–235. https://doi.org/10.1016/j.fitote.2016.01.007
Funding
Open access funding provided by Università degli Studi di Siena within the CRUI-CARE Agreement. This manuscript received no funding
Author information
Authors and Affiliations
Contributions
PG: Investigation; Methodology; Formal analysis; Writing – Original Draft. FM: Validation; Writing – Review and Edit. EM: Resources; Writing – Review and Edit. MB: Conceptualization; Validation; Writing – Review and Edit.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Governa, P., Manetti, F., Miraldi, E. et al. Effects of in vitro simulated digestion on the antioxidant activity of different Camellia sinensis (L.) Kuntze leaves extracts. Eur Food Res Technol 248, 119–128 (2022). https://doi.org/10.1007/s00217-021-03864-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03864-1