Abstract
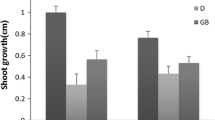
Due to extreme climate changes, plants are exposed to severe soil water content, i.e. flooding and drought. The aim of this study was to assess the effect of different soil water content on the nutritional potential of young Chinese cabbage (Brassica rapa ssp. pekinensis) on the level of phenolics, sugars, photosynthetic pigments and antioxidant capacity. Total phenolic acids were induced in plants grown under drought conditions. Both types of stress increased soluble sugars in Chinese cabbage but reduced total tannins. Plants grown under drought had more L-ascorbic acid than the control group; however, the concentration of ferulic acid and quercetin was reduced. On the other hand, excess of water increased the amount of sinapic and ferulic acid, main hydroxycinnamic acids in Chinese cabbage. Photosynthetic pigments were more susceptible to flooding (reduced amount) than drought, the only exception was chlorophyll b whose concentration was significantly higher in plants grown under drought. In addition, among all the measured parameters, chlorophyll b reacted most specifically against flooding (decreased) and drought (increased). Chinese cabbage grown under flooding had reduced amount of porphyrins compared to the one grown under normal and drought conditions, but showed higher antioxidant capacity (ABTS). Based on the results, the use of excess water could be considered as a possibility when growing young Chinese cabbage since it increased the concentration of antioxidants sinapic and ferulic acid and resulted in higher antioxidant capacity, recorded by ABTS assay, compared to control plants.


Similar content being viewed by others
References
Ashraf MA, Iqbal M, Rasheed R, Hussain I, Riaz M, Arif MS (2018) Environmental stress and secondary metabolites in plants: an overview. In: Ahmad P, Ahanger MA, Singh VP, Tripathi DK, Alam P, Alyemeni MN (eds) Plant metabolites and regulation under environmental stress, 1st edn. Academic Press, Cambridge, pp 153–167
Chen XS, Li YF, Cai YH, Xie YH, Deng ZM, Li F, Hou ZY (2019) Differential strategies to tolerate flooding in Polygonum hydropiper plants originating from low- and high-elevation habitats. Front Plant Sci 9:1970
Nouman W, Olson ME, Gull T, Zubair M, Basra SMA, Qureshi MK, Sultan MT, Shaheen M (2018) Drought affects size, nutritional quality, antioxidant activities and phenolic acids pattern of Moringa oleifera Lam. J Appl Bot Food Qual 91:79–87
Laxa M, Liebthal M, Telman W, Chibani K, Dietz KJ (2019) The role of the plant antioxidant system in drought tolerance. Antioxidants 8:94
Francisco M, Tortosa M, Martínez-Ballesta MC, Velasco P, García-Viguera C, Moreno DA (2017) Nutritional and phytochemical value of Brassica crops from the agri-food perspective. Ann Appl Biol 170:273–285
Golubkina N, Zayachkovsky V, Stepanov V, Deryagina V, Rizhova N, Kirsanov K, Caruso G (2020) High temperature and humidity effect on biochemical characteristics of organically-grown parsnip roots compared to garlic bulbs. Plant Foods Hum Nutr 75:292–297
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Howard LR, Clar JR, Brownmiller C (2003) Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J Sci Food Agric 83:1238–1247
Lo Scalzo R (2008) Organic acids influence on DPPH scavenging by ascorbic acid. Food Chem 107:40–43
Weidner S, Karolak M, Karamać M, Kosínska A, Amarowicz R (2009) Phenolic compounds and properties of antioxidants in grapevine roots (Vitis vinifera L.) under drought stress followed by recovery. Acta Soc Bot Pol 78:97–103
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Sumanta N, Haque CI, Nishika J, Suprakash R (2014) Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res J Chem Sci 4:63–69
Šola I, Vujčić Bok V, Dujmović M, Rusak G (2020) Developmentally-related changes in phenolic and L-ascorbic acid content, and antioxidant capacity of Chinese cabbage sprouts. J Food Sci Technol 57:702–712
Poljuha D, Šola I, Bilić I et al (2015) Phenolic composition, antioxidant capacity, energy content and gastrointestinal stability of Croatian wild edible plants. Eur Food Res Technol 241:573–585
Bhalodia NR, Nariya PB, Acharya RN, Shukla VJ (2013) In vitro antioxidant activity of hydroalcoholic extract from the fruit pulp of Cassia fistula Linn. Ayu 34:209–214
Stagnari F, Galieni A, Pisante M (2016) Drought stress effects on crop quality. In: Ahmad P (ed) Water stress and crop plants: a sustainable approach, 1st edn. John Wiley & Sons Ltd, New York, pp 375–392
Shawon RA, Kang BS, Lee SG, Kim SK, Lee HJ, Katrich E, Gorinstein S, Ku YG (2020) Influence of drought stress on bioactive compounds, antioxidant enzymes and glucosinolate contents of Chinese cabbage (Brassica rapa). Food Chem 308:125657
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70:1–9
Malisch CM, Salminen JP, Kölliker R, Engström MT, Suter D, Studer B, Lüscher A (2016) Drought effects on proanthocyanidins in sainfoin (Onobrychis viciifolia Scop.) are dependent on the plant’s ontogenetic stage. J Agric Food Chem 64:9307–9316
Mohammadkhani N, Heidari R (2008) Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl Sci J 3:448–453
Walter J, Hein R, Auge H, Beierkuhnlein C, Löffler S, Reifenrath K, Schädler M, Weber M, Jentsch A (2012) How do extreme drought and plant community composition affect host plant metabolites and herbivore performance? Arthropod-Plant Inte 6:15–25
Rezayian R, Niknam V, Ebrahimzadeh H (2018) Differential responses of phenolic compounds of Brassica napus under drought stress. Iran J Plant Physiol 8:2417–2425
Du Y, Zhao Q, Chen L, Yao X, Zhang W, Zhang B, Xie F (2020) Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol Biochem 146:1–12
Ismail AM, Ella ES, Vergara GV, Mackill DJ (2009) Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa L.). Ann Bot 103:197–209
Pourabdal L, Heidary R, Farboodnia T (2008) Effects of three different flooding periods on some anatomical, morphological and biochemical Shangings in maize (Zea mays L.) seedlings. Asian J Plant Sci 7:90–94
Seminario A, Song L, Zulet A, Nguyen HT, González EM, Larrainzar E (2017) Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front Plant Sci 8:1042
Robinson JM, Bunce JA (2000) Influence of drought-induced water stress on soybean and spinach leaf ascorbate-dehydroascorbate level and redox status. Int J Plant Sci 161:271–279
Kałużewicz A, Lisiecka J, Gąsecka M, Krzesiński W, Spiżewski T, Zaworska A, Frąszczak B (2017) The effects of plant density and irrigation on phenolic content in cauliflower. Hort Sci 44:178–185
Khang DT, Ha PTT, Lang NT, Tuyen PT, Minh LT, Minh TN, Bach DT, Xuan TD (2016) Involvement of phenolic compounds in anaerobic flooding germination of rice (Oryza sativa L.). Int Lett Nat Sci 56:73–81
Król A, Amarowicz R, Weidner S (2014) Changes in the composition of phenolic compounds and antioxidant properties of grapevine roots and leaves (Vitis vinifera L.) under continuous of long-term drought stress. Acta Physiol Plant 36:1491–1499
Hura T, Hura K, Grzesiak S (2008) Contents of total phenolics and ferulic acid, and PAL activity during water potential changes in leaves of maize single-cross hybrids of different drought tolerance. J Agron Crop Sci 194:104–112
Nageswara Rao RC, Talwar HS, Wright GC (2001) Rapid assessment of specific leaf area and leaf nitrogen in peanut (Arachis hypogaea L.) using a chlorophyll meter. J Agron Crop Sci 186:175–182
Massacci ASM, Nabiev L, Pietrosanti SK, Nematov TN, Chernikova KT, Leipner J (2008) Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiol Biochem 46:189–195
Soliz-Guerrero JB, de Rodriguez DJ, Rodriguez-Garcia R et al (2002) Quinoa saponins: concentration and composition analysis. In: Janick J, Whipkey A (eds) Trends in new crops and new uses. ASHS Press, Alexandria, pp 110–114
Schlemmer MR, Francis DD, Shanahan JF, Schepers JS (2005) Remotely measuring chlorophyll content in corn leaves with differing nitrogen levels and relative water content. Agron J 97:106–112
Anjum F, Yaseen M, Rasoo E et al (2003) Water stress in barley (Hordeum vulgare L.). II. Effect on chemical composition and chlorophyll contents. Pak J Agric Sci 40:45–49
Makhmudov SHA (1983) A study on chlorophyll formation in wheat leaves under moisture stress. Field Crops Abst 39:1353
Ashraf M, Mehmood S (1990) Response of four Brassica species to drought stress. Environ Exp Bot 30:93–100
Hanson PM, Yang RY, Chang LC, Ledesma L, Ledesma D (2009) Contents of carotenoids, ascorbic acid, minerals and total glucosinolates in leafy brassica pakchoi (Brassica rapa L. chinensis) as affected by season and variety. J Sci Food Agric 89:906–914
Hanson PM, Yang RY, Chang LC, Ledesma L, Ledesma D (2011) Carotenoids, ascorbic acid, minerals, and total glucosinolates in choysum (Brassica rapa cvg. parachinensis) and kailaan (B. oleraceae Alboglabra group) as affected by variety and wet and dry season production. J Food Compost Anal 24:950–962
Huang H, Ullah F, Zhou DX, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800
Floegel A, Kim DO, Chung SJ, Koo SI, Chun OK (2011) Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compost Anal 24:1043–1048
Jolliffe IT, Cadima J (2016) Principal component analysis: a review and recent developments. Phil Trans R Soc A 374:20150202
Acknowledgements
This research was financially supported by the University of Zagreb (Grant No. 20284112).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
217_2021_3759_MOESM1_ESM.docx
Supplementary Fig. S1 Hierarchical clustering of the Chinese cabbage plants grown under different water conditions as a function of their phytochemical composition and antioxidant capacity. Table S1 Pearson’s correlation coefficient (r) between the phytochemical content and antioxidant capacity of control and stressed Chinese cabbage (DOCX 118 KB)
Rights and permissions
About this article
Cite this article
Šola, I., Stić, P. & Rusak, G. Effect of flooding and drought on the content of phenolics, sugars, photosynthetic pigments and vitamin C, and antioxidant potential of young Chinese cabbage. Eur Food Res Technol 247, 1913–1920 (2021). https://doi.org/10.1007/s00217-021-03759-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03759-1