Abstract
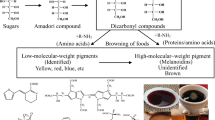
With the emergence of mechanochemistry as a fast and efficient synthetic methodology, we reported earlier that ball milling of glucose with histidine leads to the formation of reaction mixtures rich in Schiff bases. Upon subsequent thermal treatments, these mixtures exhibited enhanced reactivity generating more browning and pyrazine-rich volatiles, compared to non-milled samples. To rationalize these observations, we further investigated the mechanochemistry of the Maillard reaction using glycolaldehyde and histidine or phenylalanine as model systems. The conversion of Schiff bases into reactive 5-oxazolidinone in these model systems was proposed as the basis of the observed enhanced reactivity. Furthermore, the formation of 5-oxazolidinone in the ball-milled samples was verified through direct spectroscopic observation of its characteristic FTIR absorption band between 1780 cm−1 and 1810 cm−1 and through the identification of its specific degradation products. The important role of the carboxylic acid moiety in enabling the formation of reactive 5-oxazolidinone intermediate that facilitates its subsequent decarboxylation and formation of downstream degradation products such as dihydropyrazines and Strecker aldehydes were elucidated.






Similar content being viewed by others
References
Maillard L (1912) Action of amino acids on sugars. Formation of melanoidins in a methodical way. Compte Rend Acad Sci 154:66–68
Nursten HE (2005) The Maillard reaction: chemistry, biochemistry, and implications. R Soc Chem. https://doi.org/10.1039/9781847552570
Hodge JE (1953) Dehydrated foods, chemistry of browning reactions in model systems. J Agric Food Chem 1(15):928–943. https://doi.org/10.1021/jf60015a004
Chu FL, Yaylayan VA (2008) Post-Schiff base chemistry of the Maillard reaction: mechanism of Imine Isomerization. Ann N Y Acad Sci 1126(1):30–37. https://doi.org/10.1196/annals.1433.041
Høltermand A (1966) The browning reaction. Starch Stärke 18(10):319–328. https://doi.org/10.1002/star.19660181004
Herbst R, Engel L (1934) A reaction between α-ketonic acids and α-amino acids. J Biol Chem 107(2):505–512
Wnorowski A, Yaylayan VA (2003) Monitoring carbonyl-amine reaction between pyruvic acid and α-amino alcohols by FTIR spectroscopy a possible route to Amadori products. J Agric Food Chem 51(22):6537–6543. https://doi.org/10.1021/jf034581y
Chu FL, Yaylayan VA (2009) FTIR monitoring of oxazolidin-5-one formation and decomposition in a glycolaldehyde–phenylalanine model system by isotope labeling techniques. Carbohydr Res 344(2):229–236. https://doi.org/10.1016/j.carres.2008.10.011
Manini P, d’Ischi M, Prota G (2001) An unusual decarboxylative Maillard Reaction between l-DOPA and d-Glucose under biomimetic conditions: factors governing competition with Pictet−Spengler condensation. J Organ Chem 66(15):5048–5053. https://doi.org/10.1021/jo010078d
Manini P, Napolitano A, d’Ischia M (2005) Reactions of d-glucose with phenolic amino acids: further insights into the competition between Maillard and Pictet–Spengler condensation pathways. Carbohydr Res 340(18):2719–2727. https://doi.org/10.1016/j.carres.2005.09.021
Hayashi T, Mase S, Namiki M (1985) Formation of the N, N’-dialkylpyrazine cation radical from glyoxal dialkylimine produced on reaction of a sugar with an amine or amino acid. Agric Biol Chem 49(11):3131–3137. https://doi.org/10.1271/bbb1961.49.3131
Glomb MA, Monnier VM (1995) Mechanism of protein modification by glyoxal and glycolaldehyde, reactive intermediates of the Maillard reaction. J Biol Chem 270(17):10017–10026. https://doi.org/10.1074/jbc.270.17.10017
Xing H, Yaylayan V (2020) Mechanochemical generation of Schiff bases and Amadori products and utilization of diagnostic MS/MS fragmentation patterns in negative ionization mode for their analysis. Carbohydr Res 495:108091. https://doi.org/10.1016/j.carres.2020.108091
Xing H, Yaylayan V (2020) Investigation of thermo-chemical properties of mechanochemically generated glucose–histidine Maillard reaction mixtures. Eur Food Res Technol. https://doi.org/10.1007/s00217-020-03611-y
Koch BP, Dittmar T, Witt M, Kattner G (2007) Fundamentals of molecular formula assignment to ultrahigh resolution mass data of natural organic matter. Anal Chem 79(4):1758–1763. https://doi.org/10.1021/ac061949s
Kim S, Kramer RW, Hatcher PG (2003) Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the Van Krevelen Diagram. Anal Chem 75(20):5336–5344. https://doi.org/10.1021/ac034415p
Kaupp G, Schmeyers J, Boy J (2000) Quantitative solid-state reactions of amines with carbonyl compounds and isothiocyanates. Tetrahedron 56(36):6899–6911. https://doi.org/10.1016/S0040-4020(00)00511-1
Namiki M, Hayashi T (1983) A new mechanism of the Maillard reaction involving sugar fragmentation and free radical formation. In. ACS Publications. https://doi.org/10.1021/bk-1983-0215.ch002
Granvogl M, Beksan E, Schieberle P (2012) New insights into the formation of aroma-active Strecker aldehydes from 3-oxazolines as transient intermediates. J Agric Food Chem 60(25):6312–6322. https://doi.org/10.1021/jf301489j
Hofmann T, Bors W, Stettmaier K (1999) Studies on radical intermediates in the early stage of the nonenzymatic browning reaction of carbohydrates and amino acids. J Agric Food Chem 47(2):379–390. https://doi.org/10.1021/jf980626x
Tsuge O, Kanemasa S, Ohe M, Takenaka S (1987) Simple generation of nonstabilized azomethine ylides through decarboxylative condensation of α-amino acids with carbonyl compounds via 5-oxazolidinone intermediates. Bull Chem Soc Jpn 60(11):4079–4089. https://doi.org/10.1246/bcsj.60.4079
Aurelio L, Box JS, Brownlee RT, Hughes AB, Sleebs MM (2003) An efficient synthesis of N-methyl amino acids by way of intermediate 5-oxazolidinones. J Organ Chem 68(7):2652–2667. https://doi.org/10.1021/jo026722l
Perez Locas C, Yaylayan VA (2008) Further insight into thermally and pH-induced generation of acrylamide from glucose/asparagine model systems. J Agric Food Chem 56(15):6069–6074. https://doi.org/10.1021/jf073055u
Acknowledgements
The authors acknowledge funding for this research from Natural Sciences and Engineering Research Council of Canada (NSERC), Le Fonds de Recherche du Québec—Nature et Technologie (FRQNT), and the China Scholarship Council (CSC).
Funding
Natural Sciences and Engineering Research Council of Canada (NSERC), Le Fonds de Recherche du Québec—Nature et Technologie (FRQNT), and China Scholarship Council (CSC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xing, H., Yaylayan, V. Insight into the mechanochemistry of the Maillard reaction: degradation of Schiff bases via 5-oxazolidinone intermediate. Eur Food Res Technol 247, 1095–1106 (2021). https://doi.org/10.1007/s00217-021-03690-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03690-5