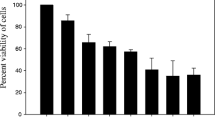
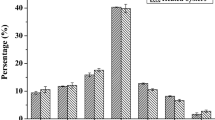
Abstract
Oyster peptides, together with polysaccharides, were found to protect the intestinal mucosal barrier against the harmful effects of 5-FU chemotherapy on rats in our previous study. However, whether oyster (Crassostrea hongkongensis) peptide can promote intestinal epithelia cell proliferation and migration alone is unknown. In this paper, oyster tissue was hydrolyzed using pepsin, trypsin, papain, bromelain, neutrase, flavorzyme, and alcalase protease. Among the hydrolysates that produced by trypsin showed the highest promoting effect on intestinal epithelia cell (IEC-6) proliferation and was fractionated into three molecular weight ultra-filtrates of > 10, 10–5, and < 5 kDa. The 10–5 kDa ultra-filtrate showed the highest promoting effect on cell proliferation, at 146.16 ± 6.56% (0.16 mg/ml), and was subjected to further purification. Two oyster peptides (F3-1 and F3-2) were purified from the 5–10 kDa ultra-filtrate by SEC and RP-HPLC. Their molecular mass and amino acid sequences were identified as VAPEEHPVLL (MW, 1102.5872 Da) and SYELPDGQVITIGNER (MW, 1789.9247 Da) by MALDI–TOF–MS/MS. Peptides F3-1 and F3-2 exhibited 150.81 ± 12.34% (5 µg/ml) and 151.73 ± 12.28% (6.25 µg/ml) promoting effects on IEC-6 proliferation, respectively, and both peptides showed greater promoting effects than the positive control (proglumide) at the same concentration under LPS stimulation culture conditions. This study indicated that oyster peptides could serve as a potential source of a functional food constituent for intestinal epithelium protection.






Similar content being viewed by others
References
Zhang HW, Xia X, Han FF, Jiang Q, Rong YL, Song DG, Wang YZ (2015) Cathelicidin-BF, a novel antimicrobial peptide from Bungarus fasciatus, attenuates disease in a dextran sulfate sodium model of colitis. Mol Pharm 12(5):1648–1661
Loddo I, Romano C (2015) Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front Immunol 6:551. https://doi.org/10.3389/fimmu.2015.00551
Wang B, Wu GY, Zhou ZG, Dai ZL, Sun YL, Ji Y, Li W, Wang WW, Liu C, Han F, Wu ZL (2015) Glutamine and intestinal barrier function. Amino Acids 47(10):2143–2154
Go¨ke M, Podolsky DK (2003) Remission, relapse, intestinal healing and repair. In: Targan SR, Shanahan F, Karp LC, Targan SR, Shanahan F, Karp LC (eds) Inflammatory bowel disease: from bench to bedside. Kluwer, Boston, pp 197–209
Schroll S, Sarlette A, Ahrens K, Manns MP, Goke M (2005) Effects of azathioprine and its metabolites on repair mechanisms of the intestinal epithelium in vitro. Regul Pept 131(1–3):1–11
Mazzuoli S, Guglielmi FW, Antonelli E, Salemme M, Bassotti G, Villanacci V (2013) Definition and evaluation of mucosal healing in clinical practice. Dig Liver Dis 45(12):969–977
Hoffmann W (2005) TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci 62(24):2932–2938
Lan A, Blachier F, Benamouzig R, Beaumont M, Barrat C, Coelho D, Lancha A, Kong XF, Yin YL, Marie JC, Tome D (2015) Mucosal healing in inflammatory bowel diseases: is there a place for nutritional supplementation? Inflamm Bowel Dis 21(1):198–207
Joo E, Yamane S, Hamasaki A, Harada N, Matsunaga T, Muraoka A, Suzuki K, Nasteska D, Fukushima T, Hayashi T, Tsuji H, Shide K, Tsuda K, Inagaki N (2013) Enteral supplement enriched with glutamine, fiber, and oligosaccharide attenuates experimental colitis in mice. Nutrition 29(3):549–555
Picanco ED, Lopes-Paulo F, Marques RG, Diestel CF, Caetano CER, de Souza MVM, Moscoso GM, Pazos HMF (2011) l-Arginine and glycine supplementation in the repair of the irradiated colonic wall of rats. Int J Colorectal Dis 26(5):561–568
Sun ZR, Liu HM, Yang ZZ, Shao DB, Zhang W, Ren Y, Sun BD, Lin JF, Xu M, Nie SN (2014) Intestinal trefoil factor activates the PI3K/Akt signaling pathway to protect gastric mucosal epithelium from damage. Int J Oncol 45(3):1123–1132
Lin JF, Sun ZR, Zhang W, Liu HM, Shao DB, Ren Y, Wen YF, Cao LP, Wolfram J, Yang ZZ, Nie SN (2015) Protective effects of intestinal trefoil factor (ITF) on gastric mucosal epithelium through activation of extracellular signal-regulated kinase 1/2 (ERK1/2). Mol Cell Biochem 404(1–2):263–270
Song DG, Zong X, Zhang HW, Wang TH, Yi HB, Luan C, Wang YZ (2015) Antimicrobial peptide cathelicidin-BF prevents intestinal barrier dysfunction in a mouse model of endotoxemia. Int Immunopharmacol 25(1):141–147
Yi HB, Yu CH, Zhang HW, Song DG, Jiang DH, Du HH, Wang YZ (2015) Cathelicidin-BF suppresses intestinal inflammation by inhibiting the nuclear factor-kappa B signaling pathway and enhancing the phagocytosis of immune cells via STAT-1 in weanling piglets. Int Immunopharmacol 28(1):61–69
Han FF, Lu ZQ, Liu YF, Xia X, Zhang HW, Wang XX, Wang YZ (2016) Cathelicidin-BF ameliorates lipopolysaccharide-induced intestinal epithelial barrier disruption in rat. Life Sci 152:199–209
Tang XP, Liu H, Yang SF, Li ZH, Zhong JF, Fang RJ (2016) Epidermal growth factor and intestinal barrier function. Mediat Inflamm 1927348. https://doi.org/10.1155/2016/1927348
Bulut K, Pennartz C, Felderbauer P, Meier JJ, Banasch M, Bulut D, Schmitz F, Schmidt WE, Hoffmann P (2008) Glucagon like peptide-2 induces intestinal restitution through VEGF release from subepithelial myofibroblasts. Eur J Pharmacol 578(2–3):279–285
Smither BR, Pang HYM, Brubaker PL (2016) Glucagon-like peptide-2 requires a full complement of Bmi-1 for its proliferative effects in the murine small intestine. Endocrinology 157(7):2660–2670
Cai BN, Pan JY, Wu YT, Wan P, Sun HL (2013) Immune functional impacts of oyster peptide-based enteral nutrition formula (OPENF) on mice: a pilot study. Chin J Oceanol Limnol 31(4):813–820
Cai BN, Chen H, Sun H, Wan P, Sun HL, Pan JY (2016) Production of immunoregulatory polysaccharides from Crassostrea hongkongensis and their positive effects as a nutrition factor in modulating the effectiveness and toxicity of 5-FU chemotherapy in mice. Food Funct 7(1):390–397
Cai BN, Wan P, Sun HL, Chen DK, Chen H, Chen X, Pan JY (2018) Protective effects of enteral nutrition supplemented with Crassostrea hongkongensis polysaccharides against 5-fluorouracil-induced intestinal mucosal damage in rats. J Med Food 21(4):348–355
Wang XQ, Zhang XW (2012) Optimal extraction and hydrolysis of Chlorella pyrenoidosa proteins. Bioresour Technol 126:307–313
Stoy ACF, Sangild PT, Skovgaard K, Thymann T, Bjerre M, Chatterton DEW, Purup S, Boye M, Heegaard PMH (2016) Spray dried, pasteurised bovine colostrum protects against gut dysfunction and inflammation in preterm pigs. J Pediatr Gastroenterol Nutr 63(2):280–287
Chen K, Xie W, Luo BY, Xiao WD, Teitelbaum DH, Yang H, Zhang KB, Zhang CJ (2014) Intestinal mucosal barrier is injured by BMP2/4 via activation of NF-kappa B signals after ischemic reperfusion. Mediat Inflamm. https://doi.org/10.1155/2014/901530
Hu LN, Fang XY, Liu HL, Gao Z, Wu XF, Sun Y, Wu XD, Xu Q (2013) Protective effects of 18 beta-glycyrrhetinic acid on LPS-induced injury in intestinal epithelial cells. Chin J Nat Med 11(1):24–29
Ruan GH, Chen ZY, Wei MP, Liu YH, Li HY, Du FY (2013) The study on microwave assisted enzymatic digestion of ginkgo protein. J Mol Catal B Enzym 94:23–28
Rowland KJ, Choi PM, Warner BW (2013) The role of growth factors in intestinal regeneration and repair in necrotizing enterocolitis. Semin Pediatr Surg 22(2):101–111
Angus DC, Wax RS (2001) Epidemiology of sepsis: an update. Crit Care Med 29(7):S109–S116
Polderman KH, Girbes ARJ (2004) Drug intervention trials in sepsis: divergent results. Lancet 363(9422):1721–1723
Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA (2001) Post-injury multiple organ failure: the role of the gut. Shock 15(1):1–10
Lee SH (2015) Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res 13(1):11–18
De-Souza DA, Greene LJ (2005) Intestinal permeability and systemic infections in critically ill patients: effect of glutamine. Crit Care Med 33(5):1125–1135
Singhal N, Kumar M, Kanaujia PK, Virdi JS (2015) MALDI–TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol 6:791. https://doi.org/10.3389/fmicb.2015.00791
Acknowledgements
We thank Dr. Yongli Gao, Shikun Dai, and Xiangyu Qin (the Equipment Public Service Center, SCSIO.CAS) for assistance in the ultra-centrifuge and mass spectrometric analyses. Our work is supported by grants from National Key R&D Program of China (No. 2018YFC0311200), Natural Science Foundation of Guangdong Province, China (No. 2018A030313088, 2018A030313903, 2018A0303130144 and 2018A030313626), the Project of Public Science and Technology Research Funds Projects of Ocean (No. 201305018), Science and Technology Program of Guangzhou, China (201707010164), the Science and Technology Planning Project of Guangdong Province, China (Nos. 2013B090800002 and 2015B090904003) and Pearl River S&T Nova Program of Guangzhou (201710010095).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflicts of interest.
Compliance with ethics requirements
The research has fully complied with research ethics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pan, J., Wan, P., Chen, D. et al. Purification and identification of intestinal mucosal cell proliferation-promoting peptides from Crassostrea hongkongensis. Eur Food Res Technol 245, 631–642 (2019). https://doi.org/10.1007/s00217-018-3186-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3186-1