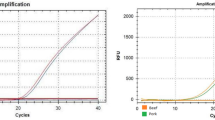
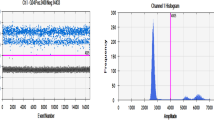
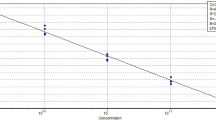
Abstract
A highly precise, quantitative method based on the droplet digital polymerase chain reaction (ddPCR) technique was developed to identify and quantify the goat and sheep content in meat products. A formula for calculating raw meat weight based on DNA copy number was established. Exclusive specificity was verified using samples from 24 different animal species, and inclusive specificity between goat and sheep was tested using five different breeds for each species. The limit of detection and the limit of quantitation for both goat and sheep were 1 and 5 copies/μL, respectively, using a cloned plasmid containing goat- and sheep-specific target DNA fragments as calibrators. The accuracy and applicability of the method were verified using mixed powder samples with known proportions of goat and sheep meat, simulate meatball samples, and commercially available products, respectively. The results confirmed that the developed ddPCR methods are highly precise for identifying and quantifying the goat and sheep meat, indicating their potential applicability in future routine analyses.


Similar content being viewed by others
References
European Commission (2002) Commission Directive 2002/86/EC. Off J Eur Commun L 305/19. Brussels
O’Mahony PJ (2013) Finding horse meat in beef products—a global problem. QJM 106:595–597
Nau JY (2013) Horse meat: first lessons of a scandal. Revue Medicale Suisse 376:532–533
Boehler P (2013) Poisoning may point to rat meat in Beijing lamb skewers. http://www.scmp.com/news/china/article/1288963/poisoning-points-rat-meat-beijing-lamb-skewers/. Accessed 15 June 14
Panwar N, Gahlot GC, Gahlot K, Ashraf M, Singh A (2015) Rapid identification of goat (Capra hircus) and sheep (Ovis aries) species in raw meat using duplex PCR assay. Indian J Anim Res 49:537–541
Cai YC, Li X, Lv R, Yang JL, Li J, He YP, Pan LW (2014) Quantitative analysis of pork and chicken products by droplet digital PCR. Biomed Res Int 2014:1–6
Floren C, Wiedemann I, Brenig B, Schütz E, Beck J (2014) Species identification and quantification in meat and meat products using droplet digital PCR (ddPCR). Food Chem 173:1054–1058
Laube I, Zagon J, Broll H (2007) Quantitative determination of commercially relevant species in foods by real-time PCR. Int J Food Sci Technol 42:336–341
Druml B, Mayer W, Cichna-Markl M, Hochegger R (2015) Development and validation of a TaqMan real-time PCR assay for the identification and quantification of roe deer (Capreolus capreolus) in food to detect food adulteration. Food Chem 178:319–326
Ballin NZ, Vogensen FK, Karlsson AH (2009) Species determination—can we detect and quantify meat adulteration? Meat Sci 83:165–174
Kumar A, Kumar RR, Sharma BD, Gokulakrishnan P, Mendiratta SK, Sharma D (2015) Identification of species origin of meat and meat products on the DNA basis: a review. Crit Rev Food Sci Nutr 55:1340–1351
Fang X, Zhang C (2016) Detection of adulterated murine components in meat products by TaqMan(c) real-time PCR. Food Chem 192:485–490
Cai YC, Wang Q, He YP, Pan LW (2017) Interlaboratory validation of a real-time PCR detection method for bovine- and ovine-derived material. Meat Sci 134:119–123
Motalib Hossain MA, Eaqub Ali Md, Abd Hamid SB, Asing Mustafa S, Mohd Desa MN, Zaidul ISM (2017) Targeting double genes in multiplex PCR for discriminating bovine, buffalo and porcine materials in food chain. Food Control 73:175–184
Motalib Hossain MA, Eaqub Ali Md, Abd Hamid SB, Asing Mustafa S, Mohd Desa MN, Zaidul ISM (2016) Double gene targeting multiplex polymerase chain reaction–restriction fragment length polymorphism assay discriminates beef, buffalo, and pork substitution in frankfurter products. J Agric Food Chem 64:6343–6354
Motalib Hossain MA, Eaqub Ali Md, Sultana S, Asing Bonny SQ, Abdul Kader Md, Rahman MA (2017) Quantitative tetraplex real-time polymerase chain reaction assay with TaqMan probes discriminates cattle, buffalo and porcine materials in food chain. J Agric Food Chem 65:3975–3985
Sanders R, Huggett JF, Bushell CA, Cowen S, Scott DJ, Foy CA (2011) Evaluation of digital PCR for absolute DNA quantification. Anal Chem 83:6474–6484
Hindson CM, Chevillet JR, Briggs HA, Gallichotte EN, Ruf IK, Hindson BJ, Vessella RL, Tewari M (2013) Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods 10:1003–1005
Zhan C, Yan L, Wang L, Jin YL, Chen L, Shi Y, Wang Q (2015) The development and application of digital PCR. Fudan Univ Sci 42:786–789
Taylor SC, Carbonneau J, Shelton DN, Boivin G (2015) Optimization of droplet digital PCR from RNA and DNA extracts with direct comparison to RT-qPCR: clinical implications for quantification of oseltamivir-resistant subpopulations. J Virol Methods 224:58–66
Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ et al (2011) High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem 83:8604–8610
Pinheiro LB, Coleman VA, Hindsonetal CM (2012) Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal Chem 84:1003–1011
Whale AS, Cowen S, Foy CA, Huggett JF (2013) Methods for applying accurate digital PCR analysis on low copy DNA samples. PLoS One 8:1–10
Morisset D, Štebih D, Milavec M, Gruden K, Žel J (2013) Quantitative analysis of food and feed samples with droplet digital PCR. PLoS One 8:1–9
Dingle TC, Sedlak RH, Cook L, Jerome KR (2013) Tolerance of droplet-digital PCR versus real-time quantitative PCR to inhibitory substances. Clin Chem 59:1670–1672
Sanmamed MF, Fernández-Landázuri S, Rodríguez C, Zárate R, Lozano MD, Zubiri L, Perez-Gracia JL, Martín-Algarra S, González A (2015) Quantitative cell-free circulating BRAFV600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin Chem 61:297–304
Rački N, Dreo T, Gutierrez-Aguirre I, Blejec A, Ravnikar M (2014) Reverse transcriptase droplet digital PCR shows high resilience to PCR inhibitors from plant, soil and water samples. Plant Methods 10:1–10
Cai YC, He YP, Lv R, Chen HC, Wang Q, Pan LW (2017) Detection and quantification of beef and pork materials in meat products by duplex droplet digital PCR. PLoS One 12:1–12
Scollo F, Egea LA, Gentile A, Malfa SL, Dorado G, Hernandez P (2016) Absolute quantification of olive oil DNA by droplet digital-PCR (ddPCR): comparison of isolation and amplification methodologies. Food Chem 213:388–394
Hu W, Chen RH, Zhang C, An ZY, Wang B, Ping Y (2014) Species identification and absolute quantification of biological samples by droplet digital PCR. Fa Yi Xue Za Zhi 30:342–345
ISO 21571 (2005) Foodstuffs—methods of analysis for the detection of genetically modified organisms and derived products—nucleic acid extraction. A.1: preparation of PCR-quality DNA using phenol/chloroform-based DNA extraction methods, pp 1–43
Lowe TM, Eddy SR (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25:955–964
Bignon C, Binart N, Ormandy C, Schuler LA, Kelly PA, Djiane J (1997) Long and short forms of the ovine prolactin receptor: cDNA cloning and genomic analysis reveal that the two forms arise by different alternative splicing mechanisms in ruminants and in rodents. J Mol Endocrinol 19:109–120
ISO 16140-2 (2016) Microbiology of the food chain—method validation—part 2: protocol for the validation of alternative (proprietary) methods against a reference method, pp 1–62
Uhlig S, Frost K, Colson B, Simon K, Mäde D, Reiting R, Gowik P, Grohmann L (2015) Validation of qualitative PCR methods on the basis of mathematical–statistical modelling of the probability of detection. Accred Qual Assur 20:75–83
Fan LL, Li P, Fu CL, Ding HL, Chen Y (2014) Detection of chicken-derived ingredients in foods by fluorescence-based quantitative real-time PCR. Food Sci 35:248–251
Zuo ZY, Sun DF (2016) Bovine derived materials and porcine derived materials detecting with real-time fluorescence PCR. Guizhou Agric Sci 44:130–132
Acknowledgements
The authors are grateful to the reviewers for their careful corrections of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Financial support from the Shanghai Science and Technology Commission Standard Special Fund (16DZ0501501), Shanghai Entry-Exit Food and Feed Safety Special Technology Service Platform Fund (17DZ2293700), Project funded by China Postdoctoral Science Foundation (2017M611628), Huaian Technical Fund (HAS201618) and Shanghai Entry-Exit Inspection and Quarantine Bureau of Science and Technology Plan Projects Fund (HK008-2017) are acknowledged with thanks.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Compliance with ethics requirements
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Wang, Q., Cai, Y., He, Y. et al. Droplet digital PCR (ddPCR) method for the detection and quantification of goat and sheep derivatives in commercial meat products. Eur Food Res Technol 244, 767–774 (2018). https://doi.org/10.1007/s00217-017-3000-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-3000-5