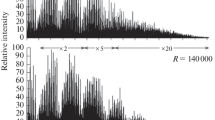
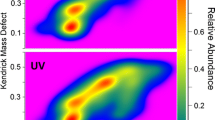
Abstract
Lignin is the second most abundant biopolymer in nature and a promising renewable feedstock for the production of aromatic compounds, composite materials, sorbents, etc. Being a complex mixture of oligomeric molecules with an irregular structure, natural lignin is an extremely difficult object to study. Its molecular level characterization requires advanced analytical techniques among which atmospheric pressure photoionization Orbitrap mass spectrometry holds a promising place. In the present study, Kendrick mass defect (KMD) analysis was proposed to facilitate the visualization and interpretation of Orbitrap mass spectra of the biopolymer on an example of Siberian pine dioxane lignin preparation. The use of the typical guaiacylpropane structure C10H12O4 as a Kendrick base unit made it possible to effectively identify oligomer series with different polymerization degrees and structurally related compounds, as well as to reliably determine the elemental compositions and structures of oligomers with high molecular weights (> 1 kDa). For the first time, KMD analysis was applied to the interpretation of the complex tandem mass spectra of lignin oligomers, rapid discrimination of the product ion series, and the establishment of the main collision-induced dissociation pathways. It was demonstrated that especially promising was the use of KMD filtering in the study of broadband fragmentation tandem mass spectra, which allows for the structural characterization of all oligomers with a particular degree of polymerization.
Graphical abstract







Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the supporting information of this article.
References
Morreel K, Kim H, Lu F, Dima O, Akiyama T, Vanholme R, Niculaes C, Goeminne G, Inzé D, Messens E, Ralph J, Boerjan W. Mass spectrometry-based fragmentation as an identification tool in lignomics. Anal Chem. 2010; 82:8095–81; https://doi.org/10.1021/ac100968g.
Banoub J, Delmas G-H, Joly N, Mackenzie G, Cachet N, Benjelloun-Mlayah B, Delmas M. A critique on the structural analysis of lignins and application of novel tandem mass spectrometric strategies to determine lignin sequencing. J Mass Spectrom. 2015; 50: 5–48; https://doi.org/10.1002/jms.3541.
Ralph J, Lundquist K, Brunow G, Lu F, Kim H, Schatz PF, Marita JM, Hatfield RD, Ralph SA, Christensen JH, Boerjan W. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochemistry Rev. 2004; 3:29–60; https://doi.org/10.1023/B:PHYT.0000047809.65444.a4.
Heitner C, Dimmel DR, Schmidt JA. Lignin and lignans: advances in chemistry. Boca Raton: CRC Press; 2010.
Abe A, Dusek K, Kobayashi S. Advances in polymer science. Springer-Verlag; 2010.
Tolbert A, Akinosho H, Khunsupat R, Naskar AK, Ragauskas AJ. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels Bioprod Biorefining. 2014; 8:836–856; https://doi.org/10.1002/bbb.1500.
Letourneau DR, Volmer DA. Mass spectrometry-based methods for the advanced characterization and structural analysis of lignin: a review. Mass Spec Rev. 2021;42(1):144–88. https://doi.org/10.1002/mas.21716.
Meija J. Mathematical tools in analytical mass spectrometry. Anal Bioanal Chem. 2006;385:486–99. https://doi.org/10.1007/s00216-006-0298-4.
Van Krevelen D. Graphical statistical method for the study of structure and reaction processes of coal. Fuel. 1950;29:228–69.
Qi Y, Hempelmann R, Volmer DA. Shedding light on the structures of lignin compounds: photo-oxidation under artificial UV light and characterization by high resolution mass spectrometry. Anal Bioanal Chem. 2016; 408:8203–8210; https://doi.org/10.1007/s00216-016-9928-7.
Podgorski DC, Hamdan R, McKenna AM, Nyadong L, Rodgers RP, Marshall AG, Cooper WT. Characterization of pyrogenic black carbon by desorption atmospheric pressure photoionization Fourier transform ion cyclotron resonance mass spectrometry. Anal Chem. 2012; 84:1281–1287; https://doi.org/10.1021/ac202166x.
Kim S, Kramer RW, Hatcher PG. Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the Van Krevelen diagram. Anal Chem. 2003; 75:5336–5344; https://doi.org/10.1021/ac034415p.
Kendrick E. A mass scale based on CH2 = 14.0000 for high resolution mass spectrometry of organic compounds. Anal Chem. 1963; 35:2146–2154; https://doi.org/10.1021/ac60206a048.
Hughey CA, Hendrickson CL, Rodgers RP, Marshall AG, Qian K. Kendrick mass defect spectrum: a compact visual analysis for ultrahigh-resolution broadband mass spectra. Anal Chem. 2001; 73:4676–4681; https://doi.org/10.1021/ac010560w.
Cho Y, Ahmed A, Islam A, Kim S. Developments in FT-ICR MS instrumentation, ionization techniques, and data interpretation methods for petroleomics. Mass Spectrom Rev. 2015; 34:248–263; https://doi.org/10.1002/mas.21438.
Sleighter RL, Hatcher PG. The application of electrospray ionization coupled to ultrahigh resolution mass spectrometry for the molecular characterization of natural organic matter. J Mass Spectrom. 2007; 42:559–574; https://doi.org/10.1002/jms.1221.
Kew W, Blackburn JWT, Clarke DJ, Uhrín D. Interactive van Krevelen diagrams − advanced visualisation of mass spectrometry data of complex mixtures. Rapid Commun. Mass Spectrom. 2017; 31:658−662; https://doi.org/10.1002/rcm.7823.
Letourneau DR, Volmer DA. Constellation: an open-source web application for unsupervised systematic trend detection in high-resolution mass spectrometry data. J Am Soc Mass Spectrom. 2022; 33:382−389; https://doi.org/10.1021/jasms.1c00371.
Fouquet TNJ. The Kendrick analysis for polymer mass spectrometry. J Mass Spectrom. 2019; 54:933–947; https://doi.org/10.1002/jms.4480.
Zheng Q, Morimoto M, Sato H, Fouquet T. Resolution-enhanced Kendrick mass defect plots for the data processing of mass spectra from wood and coal hydrothermal extracts. Fuel. 2019; 235:944–953; https://doi.org/10.1016/j.fuel.2018.08.085.
Dier TKF, Egele K, Fossog V, Hempelmann R, Volmer DA. Enhanced mass defect filtering to simplify and classify complex mixtures of lignin degradation products. Anal Chem. 2016; 88:1328–1335; https://doi.org/10.1021/acs.analchem.5b03790.
Qi YL, Hempelmann R, Volmer DA. Two-dimensional mass defect matrix plots for mapping genealogical links in mixtures of lignin depolymerisation products. Anal Bioanal Chem. 2016; 408:4835−4843; https://doi.org/10.1007/s00216-016-9598-5.
Crawford EA, Gerbig S, Spengler B, Volmer DA. Rapid fingerprinting of lignin by ambient ionization high resolution mass spectrometry and simplified data mining. Anal Chim Acta. 2017; 994:38−48; https://doi.org/10.1016/j.aca.2017.09.012.
Dier TKF, Egele K, Fossog V, Hempelmann R, Volmer DA. Enhanced mass defect filtering to simplify and classify complex mixtures of lignin degradation products. Anal Chem. 2016; 88:1328−1335; https://doi.org/10.1021/acs.analchem.5b03790.
Dier TKF, Fleckenstein M, Militz H, Volmer DA. Exploring the potential of high resolution mass spectrometry for the investigation of lignin-derived phenol substitutes in phenolic resin syntheses. Anal Bioanal Chem. 2017; 409:3441−3451; https://doi.org/10.1007/s00216-017-0282-1.
Abanoub M, Fridgen TD, Delmas M, Banoub J. Top–down lignomics analysis of the French oak lignin by atmospheric pressure photoionization and electrospray ionization quadrupole time-of-flight tandem mass spectrometry: Identification of a novel series of lignans. J Mass Spectrom. 2021; 56:1–21; https://doi.org/10.1002/jms.4676.
Terrell E, Carré V, Dufour A, Aubriet F, Le Brech Y, Garcia-Pérez M. Contributions to lignomics: stochastic generation of oligomeric lignin structures for interpretation of MALDI−FT-ICRMS results. ChemSusChem. 2020; 13:4428−4445; https://doi.org/10.1002/cssc.202000239.
Kosyakov DS, Pikovskoi II, Ul´yanovskii NV. Dopant-assisted atmospheric pressure photoionization Orbitrap mass spectrometry – an approach to molecular characterization of lignin oligomers. Anal Chim Acta. 2021; 1179:338836; https://doi.org/10.1016/j.aca.2021.338836.
Pepper JM, Baylis PET, Adler E. The isolation and properties of lignins obtained by the acidolysis of spruce and aspen woods in dioxane-water medium. Can J Chem. 1959; 37:1241−1248; https://doi.org/10.1139/v59-183.
Pikovskoi II, Kosyakov DS, Shavrina IS, Ul’yanovskii NV. Study of nettle (Urtica dióica) lignin by atmospheric pressure photoionization Orbitrap mass spectrometry. J Anal Chem. 2019; 74:1412–1420; https://doi.org/10.1134/S1061934819140090.
Lin SY, Dence CW. Methods in lignin chemistry. Springer-Verlag; 1992.
Pikovskoi II, Ul’yanovskii NV, Gorbova NS, Kosyakov DS. Study of lignin by atmospheric pressure photoionization Orbitrap mass spectrometry: effect of spectral resolution. J Anal Chem. 2021; 76:1610–1617; https://doi.org/10.1134/S1061934821140082.
Kosyakov DS, Ul’yanovskii NV, Anikeenko EA, Gorbova NS. Negative ion mode atmospheric pressure ionization methods in lignin mass spectrometry: a comparative study. Rapid Commun Mass Spectrom. 2016; 30:2099–2108; https://doi.org/10.1002/rcm.7686.
Qi Y, Volmer DA. Rapid mass spectral fingerprinting of complex mixtures of decomposed lignin: data processing methods for high resolution full-scan mass spectra. Rapid Commun Mass Spectrom. 2018; 33: 2–10; https://doi.org/10.1002/rcm.8254.
Morreel K, Kim H, Lu F, Dima O, Akiyama T, Vanholme R, Niculaes C, Goeminne G, Inzé D, Messens E, Ralph J, Boerjan W. Mass spectrometry-based fragmentation as an identification tool in lignomics. Anal Chem. 2010; 82:8095–8105; https://doi.org/10.1021/ac100968g.
Acknowledgements
This study was performed using an instrumentation of the Core Facility Center “Arktika” of the Lomonosov Northern (Arctic) Federal University.
Funding
This research was funded by Russian Science Foundation, grant number 21-73-20275.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pikovskoi, I.I., Kosyakov, D.S. Kendrick mass defect analysis — a tool for high-resolution Orbitrap mass spectrometry of native lignin. Anal Bioanal Chem 415, 3525–3534 (2023). https://doi.org/10.1007/s00216-023-04742-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04742-3