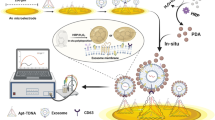
Abstract
Cardiomyocyte-derived extracellular vesicles (EVs) are a promising class of biomarkers that can advance the diagnosis of many kinds of cardiovascular diseases. Herein, we develop a new electrochemical method for the feasible detection of cardiomyocyte-derived EVs in biological fluids. The core design of the method is the fabrication of a peptide-anchored biomimetic interface consisting of a lipid bilayer and peptide probes. On the one hand, the lipid bilayer provides excellent antifouling ability to the electrode interface and facilitates the anchoring of peptide probes. On the other hand, the peptide probes equip the electrode interface with excellent binding capability and affinity to CD172a, a specific marker of cardiomyocyte-derived EVs, thus enabling the efficient and selective detection of target EVs. Taking EVs derived from the heart myoblast cells H9C2 as the model target, the method displays a wide linear detection range from 1 × 103 to 1 × 108 particles/mL with a desirable detection limit of 132 particles/mL. Furthermore, the method shows good performance in biological fluids such as serum, and thus may have great potential for practical use in the diagnosis of cardiovascular diseases.
Graphical Abstract







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article and its supplementary information file.
References
Ouyang M, Tub D, Tong L, Sarwar M, Bhimaraj A, Li C, Coté GL, Di Carlo D. A review of biosensor technologies for blood biomarkers toward monitoring cardiovascular diseases at the point-of-care. Biosens Bioelectron. 2021;171:112621. https://doi.org/10.1016/j.bios.2020.112621.
Davidson SM, Boulanger CM, Aikawa E, Badimon L, Barile L, Binder CJ, Brisson A, Buzas E, Emanueli C, Jansen F, Katsur M, Lacroix R, Lim SK, Mackman N, Mayr M, Menasché P, Nieuwland R, Sahoo S, Takov K, Thum T, Vader P, Wauben MHM, Witwer K, Sluijter JPG. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: from exosomes to microvesicles. Cardiovasc Res. 2022. https://doi.org/10.1093/cvr/cvac031.
Fu S, Zhang Y, Li Y, Luo L, Zhao Y, Yao Y. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. 2020;6:68. https://doi.org/10.1038/s41420-020-00305-y.
Zarà M, Amadio P, Campodonico J, Sandrini L, Barbieri SS. Exosomes in cardiovascular diseases Diagnostics. 2020;10:943. https://doi.org/10.3390/diagnostics10110943.
Sahoo S, Adamiak M, Mathiyalagan P, Kenneweg F, Kafert-Kasting S, Thum T. Therapeutic and diagnostic translation of extracellular vesicles in cardiovascular diseases: roadmap to the clinic. Circulation. 2021;143:1426–49. https://doi.org/10.1161/CIRCULATIONAHA.120.049254.
Zamani P, Fereydouni N, Butler AE, Navashenaq JG, Sahebkar A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trend Cardiovas Med. 2019;29:313–23. https://doi.org/10.1016/j.tcm.2018.10.010.
Min L, Wang B, Bao H, Li X, Zhao L, Meng J, Wang S. Advanced nanotechnologies for extracellular vesicle-based liquid biopsy. Adv Sci. 2021;8:2102789. https://doi.org/10.1002/advs.202102789.
Singh S, Numan A, Cinti S. Electrochemical nanobiosensors for the detection of extracellular vesicles exosomes: from the benchtop to everywhere? Biosens Bioelectron. 2022;216:114635. https://doi.org/10.1016/j.bios.2022.114635.
Feng Q, Fan W, Ren W, Liu C. Recent advances in exosome analysis assisted by functional nucleic acid-based signal amplification technologies. TrAC Trend Anal Chem. 2022;149:116549. https://doi.org/10.1016/j.trac.2022.116549.
Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118:1917–50. https://doi.org/10.1021/acs.chemrev.7b00534.
Kaya SI, Ozcelikay G, Mollarasouli F, Bakirhan NK, Ozkan SA. Recent achievements and challenges on nanomaterial based electrochemical biosensors for the detection of colon and lung cancer biomarkers. Sens Actuator B-Chem. 2022;351:130856. https://doi.org/10.1016/j.snb.2021.130856.
Bakirhan NK, Topal BD, Ozcelikay G, Karadurmus L, Ozkan SA. Current advances in electrochemical biosensors and nanobiosensors. Crit Rev Anal Chem. 2022;52:519–34. https://doi.org/10.1080/10408347.2020.1809339.
Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–5. https://doi.org/10.1126/science.12295.
Li C, Huang Y, Yang Y. Coupling of an antifouling and reusable nanoplatform with catalytic hairpin assembly for highly sensitive detection of nucleic acids using zeta potential as signal readout. Sens Actuator B-Chem. 2021;326:128845. https://doi.org/10.1016/j.snb.2020.128845.
Li H, Dauphin-Ducharme P, Arroyo-Curras N, Tran CH, Vieira PA, Li SG, Shin C, Somerson J, Kippin TE, Plaxco KW. A biomimetic phosphatidylcholine-terminated monolayer greatly improves the in vivo performance of electrochemical aptamer-based sensors. Angew Chem Int Ed. 2017;56:7492–5. https://doi.org/10.1002/anie.201700748.
McKeating KS, Hinman SS, Rais NA, Zhou ZG, Cheng Q. Antifouling lipid membranes over protein A for orientation-controlled immunosensing in undiluted serum and plasma. ACS Sens. 2019;4:1774–82. https://doi.org/10.1021/acssensors.9b00257.
Dong G, An Y, Yan P, Wu J, Li C, Liu T. A zeta potential-based homogeneous assay for amplified detection of telomerase in cancer cells. Sens Actuator B-Chem. 2022;350:130881. https://doi.org/10.1016/j.snb.2021.130881.
Manna SL, Di Natale C, Onesto V, Marasco D. Self-assembling peptides: from design to biomedical applications. Int J Mol Sci. 2021;22:12662. https://doi.org/10.3390/ijms222312662.
Loew M, Springer R, Scolari S, Altenbrunn F, Seitz O, Liebscher J, Huster D, Herrmann A, Arbuzova A. Lipid domain specific recruitment of lipophilic nucleic acids: a key for switchable functionalization of membranes. J Am Chem Soc. 2010;132:16066–72. https://doi.org/10.1021/ja105714r.
Zhang P, Cui Y, Anderson CF, Zhang C, Li Y, Wang R, Cui H. Peptide-based nanoprobes for molecular imaging and disease diagnostics. Chem Soc Rev. 2018;47:3490–529. https://doi.org/10.1039/C7CS00793K.
Anselmo A, Frank D, Papa L, Anselmi CV, Di Pasquale E, Mazzola M, Panico C, Clemente F, Soldani C, Pagiatakis C, Hinkel R, Thalmann R, Kozlik-Feldmann R, Miragoli M, Carullo P, Vacchiano M, Chaves-Sanjuan A, Santo N, Losi MA, Ferrari MC, Puca AA, Christiansen V, Seoudy H, Freitag-Wolf S, Frey N, Dempfle A, Mercola M, Esposito G, Briguori C, Kupatt C, Condorelli G. Myocardial hypoxic stress mediates functional cardiac extracellular vesicle release. Eur Heart J. 2021;42:2780–92. https://doi.org/10.1093/eurheartj/ehab247.
Tang Y, Dai Y, Huang X, Li L, Han B, Cao Y, Zhao J. Self-assembling peptide-based multifunctional nanofibers for electrochemical identification of breast cancer stem-like cells. Anal Chem. 2019;91:7531–7. https://doi.org/10.1021/acs.analchem.8b05359.
Zhang J, Chen H, Cao Y, Feng C, Zhu X, Li G. Design nanoprobe based on its binding with amino acid residues on cell surface and its application to electrochemical analysis of cells. Anal Chem. 2019;91:1005–10. https://doi.org/10.1021/acs.analchem.8b04247.
Sha L, Bo B, Yang F, Li J, Cao Y, Zhao J. Programmable DNA-fueled electrochemical analysis of lung cancer exosomes. Anal Chem. 2022;94:8748–55. https://doi.org/10.1021/acs.analchem.2c01318.
Cao Y, Wang Y, Yu X, Jiang X, Li G, Zhao J. Identification of programmed death ligand-1 positive exosomes in breast cancer based on DNA amplification-responsive metal-organic frameworks. Biosens Bioelectron. 2020;166:112452. https://doi.org/10.1016/j.bios.2020.112452.
Miao P, Ma X, Xie L, Tang Y, Sun X, Wen Z, Wang Z. Tetrahedral DNA mediated direct quantification of exosomes by contact-electrification effect. Nano Energy. 2022;92:106781. https://doi.org/10.1016/j.nanoen.2021.106781.
Zhang H, Qiao B, Guo Q, Jiang J, Cai C, Shen J. A facile and label-free electrochemical aptasensor for tumour-derived extracellular vesicle detection based on the target-induced proximity hybridization of split aptamers. Analyst. 2020;145:3557–63. https://doi.org/10.1039/D0AN00066C.
Sabaté del Río J, Woo HK, Park J, Ha HK, Kim JR, Cho YK. SEEDING to enable sensitive electrochemical detection of biomarkers in undiluted biological samples. Adv Mater. 2022;34:2200981. https://doi.org/10.1002/adma.202200981.
Lee M, Park SJ, Kim G, Park C, Lee MH, Ahn JH, Lee T. A pretreatment-free electrical capacitance biosensor for exosome detection in undiluted serum. Biosens Bioelectron. 2022;199:113872. https://doi.org/10.1016/j.bios.2021.113872.
Funding
This work was supported by the grant from Sichuan Medical Association (No. Q17041).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by YZ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Clinical experiment was approved by the Ethics Committee of People’s Hospital of Leshan and performed according to ethical standards.
Competing interests
The authors declare no competing interests.
Source of biological material
Serum samples were collected from AMI patients and control donors with informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection Advances in Extracellular Vesicle Analysis with guest editors Lucile Alexandre, Jiashu Sun, Myriam Taverna, and Wenwan Zhong.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Zhao, F., Zheng, B. et al. Peptide-anchored biomimetic interface for electrochemical detection of cardiomyocyte-derived extracellular vesicles. Anal Bioanal Chem 415, 1305–1311 (2023). https://doi.org/10.1007/s00216-022-04419-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04419-3