Abstract
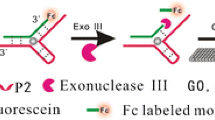
Ultrasensitive and specific detection of cocaine is of great significance for monitoring cocaine abuse. Herein, a fluorescent aptasensor via coupling CRISPR-Cas12a, with magnetic nanoparticles (MNPs), split-aptamer, and terminal deoxynucleotidyl transferase (TdT), was developed for the detection of cocaine. In short, the complete cocaine aptamer is split into two parts, one is modified on magnetic nanoparticles (MNPs) and the other is free. The presence of cocaine will mediate the binding of these two segments. Then TdT will mediate the extension to form an ultra-long sequence that can bind with multiple CRISPR-Cas12a resulting in the trans-cleavage activity of CRISPR-Cas12a being triggered. Thence, the DNA reporter which is bi-labeled with fluorophore and quencher is cleaved resulting in the generation of a fluorescence signal. The developed fluorescent aptasensor realizes the detection of cocaine with excellent sensitivity and specificity. The detection limit is low down to 33 pM, and the linear range is from 330 to 1.65 × 105 pM. Most importantly, this fluorescent aptasensor can be successfully applied to the determination of cocaine in human plasma samples.





Similar content being viewed by others
References
Ryan SA. Cocaine use in adolescents and young adults. Pediatr Clin North Am. 2019;66(6):1135–47.
Thornton C, Grad E, Yaka R. The role of mitochondria in cocaine addiction. Biochem J. 2021;478(4):749–64.
Taghdisi SM, Danesh NM, Ramezani M, Emrani AS, Abnous K. A novel electrochemical aptasensor based on Y-shape structure of dual-aptamer-complementary strand conjugate for ultrasensitive detection of myoglobin. Biosens Bioelectron. 2016;80:532–7.
Kawa AB, Allain F, Robinson TE, Samaha AN. The transition to cocaine addiction: the importance of pharmacokinetics for preclinical models. Psychopharmacology. 2019;236(4):1145–57.
Mandrioli R, Mercolini L, Protti M. Blood and plasma volumetric absorptive microsampling (VAMS) coupled to LC-MS/ms for the forensic assessment of cocaine consumption. Molecules. 2020;25(5):1046.
Liu CG, Wang Y, Liu P, Yao QL, Zhou YY, Li CF, et al. Aptamer-T cell targeted therapy for tumor treatment using sugar metabolism and click chemistry. ACS Chem Biol. 2020;15(6):1554–65.
Perret G, Boschetti E. Aptamer-based affinity chromatography for protein extraction and purification. Adv Biochem Eng Biotechnol. 2020;174:93–139.
Zhang F, Liu J. Interactions of the cocaine and quinine aptamer with gold nanoparticles under the dilute biosensor and concentrated NMR conditions. Langmuir. 2021;37(40):11939–47.
Wu Z, Zhou H, Han Q, Lin X, Han D, Li X. A cost-effective fluorescence biosensor for cocaine based on a “mix-and-detect” strategy. Analyst. 2020;145(13):4664–70.
Su F, Zhang S, Ji H, Zhao H, Tian JY, Liu CS, et al. Two-dimensional zirconium-based metal-organic framework nanosheet composites embedded with Au nanoclusters: a highly sensitive electrochemical aptasensor toward detecting cocaine. ACS Sens. 2017;2(7):998–1005.
Oroval M, Coronado-Puchau M, Langer J, Sanz-Ortiz MN, Ribes Á, Aznar E, et al. Surface enhanced raman scattering and gated materials for sensing applications: the ultrasensitive detection of mycoplasma and cocaine. Chemistry. 2016;22(38):13488–95.
Wang L, Musile G, McCord BR. An aptamer-based paper microfluidic device for the colorimetric determination of cocaine. Electrophoresis. 2018;39(3):470–5.
Chen Z, Tan Y, Xu K, et al. Stimulus-response mesoporous silica nanoparticle-based chemiluminescence biosensor for cocaine determination. Biosens Bioelectron. 2016;75:8–14.
Qian W, Miao Z, Zhang XJ, Yang XT, Tang YY, Tang YY, et al. Functionalized reduced graphene oxide with aptamer macroarray for cancer cell capture and fluorescence detection. Mikrochim Acta. 2020;187(7):407.
Makarova KS, Wolf YI, Iranzo J, Shmakov SA, Alkhnbashi OS, Brouns SJJ, et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol. 2020;18(2):67–83.
Manghwar H, Lindsey K, Zhang X, Jin S. CRISPR/Cas system: recent advances and future prospects for genome editing. Trends Plant Sci. 2019;24(12):1102–25.
Liang M, Li Z, Wang W, Liu J, Liu L, Zhu G, et al. A CRISPR-Cas12a-derived biosensing platform for the highly sensitive detection of diverse small molecules. Nat Commun. 2019;10(1):3672.
Yu P, Yang T, Zhang D, Xu L, Cheng X, Ding S, et al. An all-in-one telomerase assay based on CRISPR-Cas12a trans-cleavage while telomere synthesis. Anal Chim Acta. 2021;1159:338404.
Li SY, Cheng QX, Liu JK, Nie XQ, Zhao GP, Wang J. CRISPR-Cas12a has both cis- and trans-cleavage activities on single-stranded DNA. Cell Res. 2018;28(4):491–3.
Xiong Y, Zhang J, Yang Z, Mou Q, Ma Y, Xiong Y, et al. Functional DNA regulated CRISPR-Cas12a sensors for point-of-care diagnostics of non-nucleic-acid targets. J Am Chem Soc. 2020;142(1):207–13.
Zhao Q, Piao J, Peng W, Wang Y, Zhang B, Gong X, et al. Simple and sensitive quantification of microRNAs via PS@Au microspheres-based DNA probes and DSN-assisted signal amplification platform. ACS Appl Mater Interfaces. 2018;10(4):3324–32.
Yu W, Li J, Zuo C, Tao Y, Bai S, Li J, et al. Specific discrimination and universal signal amplification for RNA detection by coupling toehold exchange with RCA through nucleolytic conversion of a structure-switched hairpin probe. Anal Chim Acta. 2019;1068:96–103.
Lei S, Liu Z, Xu L, Zou L, Li G, Ye B. A “signal-on” electrochemical biosensor based on DNAzyme-driven bipedal DNA walkers and TdT-mediated cascade signal amplification strategy. Anal Chim Acta. 2020;1100:40–6.
Jiang J, Lin X, Ding D, Diao G. Enzyme-free homogeneous electrochemical biosensor for DNA assay using toehold-triggered strand displacement reaction coupled with host-guest recognition of Fe3O4@SiO2@β-CD nanocomposites. Biosens Bioelectron. 2018;114:37–43.
Zou Y, Zhang H, Wang Z, Liu Q, Liu Y. A novel ECL method for histone acetyltransferases (HATs) activity analysis by integrating HCR signal amplification and ECL silver clusters. Talanta. 2019;198:39–44.
Li X, Du Z, Lin S, Tian J, Tian H, Xu W. ExoIII and TdT dependent isothermal amplification (ETDA) colorimetric biosensor for ultra-sensitive detection of Hg2. Food Chem. 2020;316:126303.
Chen J, Zeng L. Enzyme-amplified electronic logic gates based on split/intact aptamers. Biosens Bioelectron. 2013;42:93–9.
Wang Q, Liu J, Zeng J, Yang Z, Ran F, Wu L, et al. Determination of miRNA derived from exosomes of prostate cancer via toehold-aided cyclic amplification combined with HRP enzyme catalysis and magnetic nanoparticles. Anal Biochem. 2021;630:114336.
Can F, Ökten HE, Ergön-Can T, Ergenekon P, Özkan M, Erhan E. Thermodynamically designed target-specific DNA probe as an electrochemical hybridization biosensor. Bioelectrochemistry. 2020;135:107553.
Wang J, Hou J, Zhang H, Tian Y, Jiang L. Single nanochannel-aptamer-based biosensor for ultrasensitive and selective cocaine detection. ACS Appl Mater Interfaces. 2018;10(2):2033–9.
Chen Z, Lu M. Target-responsive aptamer release from manganese dioxide nanosheets for electrochemical sensing of cocaine with target recycling amplification. Talanta. 2016;160:444–8.
Emrani AS, Danesh NM, Ramezani M, Taghdisi SM, Abnous K. A novel fluorescent aptasensor based on hairpin structure of complementary strand of aptamer and nanoparticles as a signal amplification approach for ultrasensitive detection of cocaine. Biosens Bioelectron. 2016;79:288–93.
Adegoke O, Pereira-Barros MA, Zolotovskaya S, Abdolvand A, Daeid NN. Aptamer-based cocaine assay using a nanohybrid composed of ZnS/Ag2Se quantum dots, graphene oxide and gold nanoparticles as a fluorescent probe. Mikrochim Acta. 2020;187(2):104.
Abnous K, Danesh NM, Ramezani M, Taghdisi SM, Emrani AS. A novel colorimetric aptasensor for ultrasensitive detection of cocaine based on the formation of three-way junction pockets on the surfaces of gold nanoparticles. Anal Chim Acta. 2018;1020:110–5.
Chen Z, Tan Y, Xu K, Zhang L, Qiu B, Guo L, et al. Stimulus-response mesoporous silica nanoparticle-based chemiluminescence biosensor forcocaine determination. Biosens Bioelectron. 2016;75:8–14.
Funding
This work was supported by the Sanming Project of Medicine in Shenzhen (Grant No. SZZYSM202106004), Baoan District Medical and Health Basic Research Project (Grant No. 2021JD143), the Science and Technology Key Program of Shiyan (Grant No. 2021Y76), Science and Technology Key Program of Shiyan (Grant No. 21Y48), and Foundation of Health Commission of Hubei Province (Grant Nos. WJ2021F052, WJ2021M062, and WJ2021M063).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
Informed consent was obtained from all participants in the clinical trial and the protocols were approved by the Shenzhen Baoan Authentic TCM Therapy Hospital’s Ethics Committee.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, T., Liu, J., Chen, G. et al. The fluorescent aptasensor based on CRISPR-Cas12a combined with TdT for highly sensitive detection of cocaine. Anal Bioanal Chem 414, 7291–7297 (2022). https://doi.org/10.1007/s00216-022-04280-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-04280-4