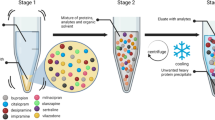
Abstract
Liquid chromatography tandem mass spectrometry (LC-MS/MS) is used routinely in clinical diagnostics; however, automating the sample pretreatment is challenging. We established and evaluated an automated method based on the magnetic bead extraction principle (MBE) to measure normetanephrine (NMN), metanephrine (MN), and 3-methoxytyramine (3-MT). The target analytes were extracted, purified, and concentrated using different solvents and chemical bond–modified magnetic beads transferred via a magnetic bar. The linearity, recovery, matrix effect, and precision of MBE were evaluated thoroughly, and compared with traditional solid-phase extraction (SPE) using 131 plasma samples. The chromatography peaks of metanephrines and 3-MT, extracted via MBE, are symmetrical, without interfering peaks. The linearity was excellent with correlation coefficient (r) > 0.99. The MBE exhibited good reproducibility with within-run coefficient variations (CVs) of 1.96–2.00%, 4.06–5.75%, and 3.89–4.90% for MN, NMN, and 3-MT, respectively. The total CVs for MN, NMN, and 3-MT were 1.96–2.80%, 5.12–5.75%, and 5.44–6.27%, respectively. The relative recoveries for MN, NMN, and 3-MT varied between 93.5 and 107.4%, whereas their biases were all within 10%. The results for MN, NMN, and 3-MT extracted via MBE compared with SPE exhibited excellent correlation, with r > 0.99; the mean bias% for MN, NMN, and 3-MT were small (−2.9%, −3.2%, and −3.2%, respectively). In conclusion, the automated MBE method for measuring plasma metanephrines and 3-MT can be applied in future routine clinical diagnostics, and the MBE principle may indicate a new era for LC-MS/MS in clinical application.



Similar content being viewed by others
References
Shi J, Dhaliwal P, Zheng Y Zi, Straseski JA, Cervinski MA, Shajani-Yi Z, et al. An intact ACTH LC-MS/MS assay as an arbiter of clinically discordant immunoassay results. Clin Chem. 2019; 10.1373/clinchem.2019.306365
Kane J, Middle J, Cawood M. Measurement of serum testosterone in women; what should we do? Ann Clin Biochem. 2007. https://doi.org/10.1258/000456307779595896.
Wright MJ, Thomas RL, Stanford PE, Horvath AR. Multiple reaction monitoring with multistage fragmentation (MRM3) detection enhances selectivity for LC-MS/MS analysis of plasma free metanephrines. Clin Chem. 2015. https://doi.org/10.1373/clinchem.2014.233551.
Lagerstedt SA, O'Kane DJ, Singh RJ. Measurement of plasma free metanephrine and normetanephrine by liquid chromatography-tandem mass spectrometry for diagnosis of pheochromocytoma. Clin Chem. 2004;https:// doi:https://doi.org/10.1373/clinchem.2003.024703
Virag D, Kiraly M, Drahos L, Edes AE, Gecse K, Bagdy G, et al. Development, validation and application of LC-MS/MS method for quantification of amino acids, kynurenine and serotonin in human plasma. J Pharm Biomed Anal. 2020. https://doi.org/10.1016/j.jpba.2019.113018.
Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014. https://doi.org/10.1210/jc.2014-1498.
Stanczyk FZ, Clarke NJ. Advantages and challenges of mass spectrometry assays for steroid hormones. J Steroid Biochem Mol Biol. 2010. https://doi.org/10.1016/j.jsbmb.2010.05.001.
Vogeser M, Kirchhoff F. Progress in automation of LC-MS in laboratory medicine. Clin Biochem. 2011. https://doi.org/10.1016/j.clinbiochem.2010.06.005.
van Faassen M, Bischoff R, Eijkelenkamp K, de Jong WHA, van der Ley CP, Kema IP. In matrix derivatization combined with LC-MS/MS results in ultrasensitive quantification of plasma free metanephrines and catecholamines. Anal Chem. 2020. doi:https://doi.org/10.1021/acs.analchem.0c01263
Yu S, Yin Y, Li Q, Yu J, Liu W, Wang D, et al. Validation of an improved liquid chromatography tandem mass spectrometry method for rapid and simultaneous analysis of plasma catecholamine and their metabolites. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1129: 121805. https://doi.org/10.1016/j.jchromb.2019.121805.
Chen H, Sippel RS, O'Dorisio MS, Vinik AI, Lloyd RV, Pacak K. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas. 2010. https://doi.org/10.1097/MPA.0b013e3181ebb4f0
Wayne P (2014) Liquid chromatography-mass spectrometry methods; Approved Guidelines. CLSI. Clinical and Laboratory Standards Institute;CLSI document C62-A.
Peitzsch M, Prejbisz A, Kroiss M, Beuschlein F, Arlt W, Januszewicz A, et al. (2013) Analysis of plasma 3-methoxytyramine, normetanephrine, and metanephrine by ultraperformance liquid chromatography-tandem mass spectrometry: utility for diagnosis of dopamine-producing metastatic phaeochromocytoma. Ann Clin Biochem. https://doi.org/10.1258/acb.2012.012112
Wayne,CLSI (2005) CLSI document EP15-A2. User verification of performance for precision and trueness ag, 23d, CLSI
Matuszewski BK, Constanzer ML, Chavez-Eng CM (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. https://doi.org/10.1021/ac020361s
Yadav M, Trivedi V, Upadhyay V, Shah G, Baxi GA, Goswami S, et al. Comparison of extraction procedures for assessment of matrix effect for selective and reliable determination of atazanavir in human plasma by LC-ESI-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. https://doi.org/10.1016/j.jchromb.2011.12.031
NCCLS (2002) Method comparison and bias estimation using patient samples; Approved Guideline—Second Edition. NCCLS document EP9-A2
Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012. https://doi.org/10.1016/j.ejca.2011.07.016.
de Jong WH, Graham KS, van der Molen JC, Links TP, Morris MR, Ross HA, et al. (2007) Plasma free metanephrine measurement using automated online solid-phase extraction HPLC tandem mass spectrometry. Clin Chem. https://doi.org/10.1373/clinchem.2007.087114
Seger C, Salzmann L. After another decade: LC-MS/MS became routine in clinical diagnostics. Clin Biochem. 2020. https://doi.org/10.1016/j.clinbiochem.2020.03.004.
Funding
This work was funded by the National Key Research and Development Program of China (No. 2021YFC2401100); Innovation-Led Industrial Cluster Project of Zheng-Luo-Xin National Innovation Demonstration Area (201200211100); and Beijing Natural Science Foundation (No.7212087).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, material preparation, and data collection and analysis. The first draft of the manuscript was written by Songlin Yu and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of Peking Union Medical College & Chinese Academy of Medical Sciences, Peking Union Medical College Hospital (JS-2956)
Conflict of interest
The authors declare no competing interests.
Source of biological material
Plasma for the method validation and comparison was obtained from that remaining from patient samples in the Department of Laboratory Medicine, Peking Union Medical College Hospital.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, S., Zhou, W., Yu, J. et al. An automated magnetic bead extraction method for measuring plasma metanephrines and 3-methoxytyramine using liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 414, 3541–3549 (2022). https://doi.org/10.1007/s00216-022-03984-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-022-03984-x